This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Embryoids shed light on a complex genetic mechanism
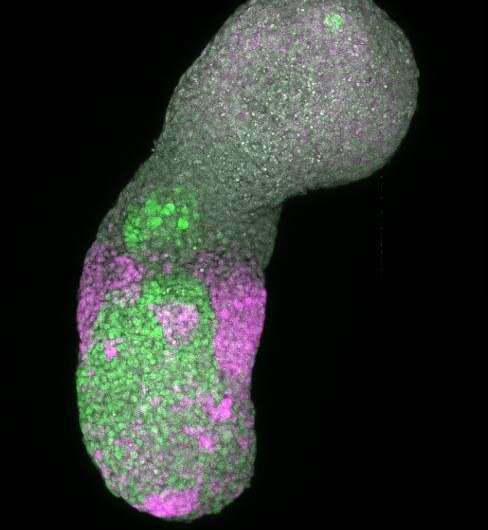
Researchers from EPFL and the University of Geneva (UNIGE) have gained new insights into a mechanism regulating the early-stage development of mouse embryos. Instead of using an animal model, the team carried out their research on pseudo-embryos grown in the lab from stem cells.
Cold cases aren't just the preserve of criminology. In science, too, there are unsolved mysteries locked away in a drawer awaiting new evidence. And just as the advent of DNA fingerprinting has helped crack old criminal cases, so new cell models are giving scientists the tools to revisit research questions that couldn't be answered with animal models alone.
Prof. Denis Duboule—who runs EPFL's Laboratory of Developmental Genomics and is also a professor at The Collège France, in Paris—knows a thing or two about this subject. For more than 30 years, he's studied the mouse genome in a quest to understand the fundamental mechanisms regulating mammal development. He's incredibly excited about the opportunities presented by "pseudo-embryos," also known as embryoids.
Because these cell models, which are cultured in vitro from stem cells, are structured and develop in a similar way to embryos, they hold immense promise for furthering our understanding of embryogenesis, or the process of embryonic development. On 15 June, a team from Duboule's lab published a paper in Nature Genetics. It presented the results of the first study in Duboule's career carried out without using an animal model.
The internal clock regulating embryo development
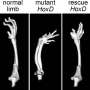
The early mammalian embryo develops along the anterior-posterior axis: the head develops first, followed by the rest of the body in "stages," moving down the axis toward the tail. In humans, a new stage develops every five hours; in mice, this time is shortened to 90 minutes. Researchers at Duboule's lab have long sought to understand how Hox architect genes—which confer an identity on each of these stages (such as a neck vertebra, or the nascent tail in mice)—are activated according to a precise schedule via an internal clock.
"We'd always wondered how a mechanism that imposes this kind of timing system on linear strands of DNA could have evolved naturally," says Duboule. "It works like a transistor that, in mice, emits a signal every 90 minutes. We spent 25 years trying to understand this phenomenon using animal models."
The problem is that this mechanism kicks into action after the embryo has implanted in the uterine wall, which makes it especially hard for researchers to observe. "At this stage, the embryo is so small that we can't yet locate it in the uterus," adds Duboule. "We'd never really found uniform material in which we could observe what was happening."
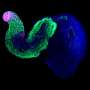
That all changed a decade ago with the emergence of embryoids—cell structures that lack the features necessary to develop into fully grown living organisms. Hocine Rekaik, a researcher at Duboule's lab and the lead author of the paper published last week, took embryoids and enriched them to obtain the part of the structure that manufactures these "stages." The result was a simplified but highly realistic cell model.
Duboule explains, "On a DNA segment, the CTCF protein acts as a sort of blocker, delaying the expression of the Hox gene located behind it. The pressure that triggers the activation signal comes from cohesin, a protein complex. Hocine developed animations where we could see this process happening in the chromatin (the structure containing DNA)—something largely impossible with a real embryo because the system becomes increasingly complex and disorderly with the passage of time. But the cells in these embryoids are heavily concentrated in the posterior section, making everything much more uniform. That means we can watch the mechanism as it plays out."
Promising new methods
Duboule is particularly pleased with the new model his team developed—not only because of the promise it holds for future research, but also because it's relatively quick and easy to use, and because it's cheaper than equivalent animal models. He's also relieved to have found a genuine alternative to mice.
"We've used a lot of animals in my lab, so I'm very happy to see the emergence of alternative models as I approach the end of my career," he says. "I don't think we're yet at the stage where we can dispense entirely with animals in pure research, but promising new methods are coming to the fore in some areas. We're entering a new era where we can produce in vitro biological models that are so realistic that, in some cases, we won't necessarily have to resort to using animals. I think that, in the medium term, we'll see a lot of pure research taking place without animal models."
At EPFL, research groups are increasingly embracing so-called alternative methods such as organoids—multicellular micro-tissues grown from stem cells that imitate the structure and function of some human organs. These methods are revolutionizing basic research, which aims to build a precise picture of how particular mechanisms function. But they're less useful in drug development research, where scientists aim to understand how a molecule affects a given system. In cases like these, animal models still have an indispensable role to play.
More information: Hocine Rekaik et al, Sequential and directional insulation by conserved CTCF sites underlies the Hox timer in stembryos, Nature Genetics (2023). DOI: 10.1038/s41588-023-01426-7
Journal information: Nature Genetics
Provided by Ecole Polytechnique Federale de Lausanne