This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A compound from fruit flies could lead to new antibiotics
Scientists at the University of Illinois Chicago have found that a peptide from fruit flies could lead to new antibiotics.
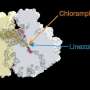
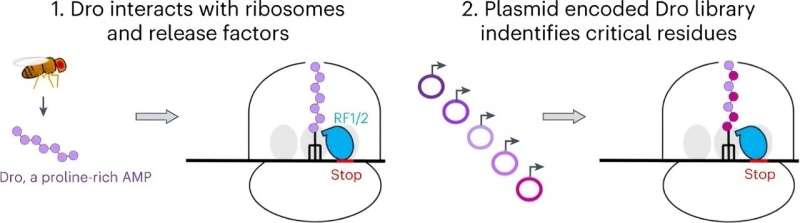
Their research, which is published in Nature Chemical Biology, shows that the natural peptide, called drosocin, protects the insect from bacterial infections by binding to ribosomes in bacteria. Once bound, drosocin prevents the ribosome from correctly completing its primary task—making new proteins, which cells need to function.
Protein production can be halted by interfering with different stages of translation—the process by which DNA is "translated" into protein molecules. The UIC scientists discovered that drosocin binds to the ribosome and inhibits translation termination when the ribosome reaches the stop signal at the end of the gene.
"Drosocin is only the second peptide antibiotic known to stop translation termination," said Alexander Mankin, study author and Distinguished Professor from the Center for Biomolecular Sciences and the department of pharmaceutical sciences in the College of Pharmacy. The other, called apidaecin and found in honeybees, was first described by UIC scientists in 2017.
The UIC lab, which is co-run by Mankin and Nora Vázquez-Laslop, research professor in the College of Pharmacy, managed to produce the fruit fly peptide and hundreds of its mutants directly in bacterial cells.
"Drosocin and its active mutants made inside the bacteria forced bacterial cells to self-destruct," Mankin said.
While the drosocin and apidaecin peptides work the same way, the researchers found that their chemical structures and the ways they bind to the ribosome are different.
"By understanding how these peptides work, we hope to leverage the same mechanism for potential new antibiotics. Comparing side-by-side the components of the two peptides facilitates engineering new antibiotics that take the best from each," Mankin said.
More information: Kyle Mangano et al, Inhibition of translation termination by the antimicrobial peptide Drosocin, Nature Chemical Biology (2023). DOI: 10.1038/s41589-023-01300-x , www.nature.com/articles/s41589-023-01300-x
Journal information: Nature Chemical Biology
Provided by University of Illinois at Chicago