This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Better understanding the bonds between carbon group elements
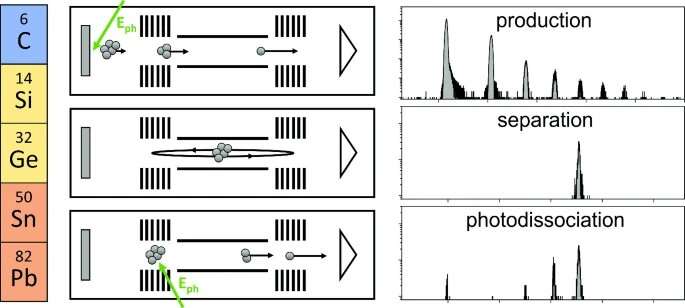
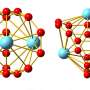
The bonds between clusters of elements in the fourteenth group of the periodic table are known to be fickle. Ranging from the nonmetal carbon, to the metalloids silicon and germanium, to the metals tin and lead, all these elements share the same configuration of valence electrons—electrons in their atoms' outermost energy level.
However, clusters formed from these elements respond differently to being excited with laser pulses. Studying the response of atomic clusters to photoexcitation as a function of the element they are composed of and their number of atoms reveals patterns that can be used to gain insight into their structure and binding mechanisms.
In a new paper in the European Physical Journal D, Paul Fischer and colleagues from the University of Greifswald in Germany reveal that, generally, the atomic bonds of carbon group clusters change from covalent to metallic when moving down the group.
To determine this, the researchers irradiated a solid target in vacuum using a pulsed laser, producing neutral and charged gas-phase atoms and clusters. Storing the charged particles in an electrostatic ion trap composed of two opposing ion-optical mirrors made it possible to separate them according to their mass-to-charge ratio with high resolution. The researchers then used a second pulsed laser to excite selected chemical species, revealing how the bonds across the group differ.
Applying this approach to more complex chemical species, such as doped or compound clusters, could lead to further insight into even more advanced binding mechanisms.
Because of its success in revealing subtle properties of cluster bonds in the well-studied carbon group, the authors conclude their method has the potential to unlock hidden properties of lesser-studied clusters.
More information: Paul Fischer et al, Photodissociation of small group-14 atomic clusters in a multi-reflection time-of-flight mass spectrometer, The European Physical Journal D (2023). DOI: 10.1140/epjd/s10053-023-00607-7
Journal information: European Physical Journal D
Provided by Springer