This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Unraveling the mysteries of p62-bodies and the cellular recycling pathway
Our body functioning is delicately balanced between the synthesis and breakdown of various cellular components. When these cellular components grow old or get damaged, they are digested by a process called autophagy—literally, "self-eating." This process not only helps in the elimination of toxic wastes, but also helps to deliver building blocks for the synthesis of new cellular macromolecules. Thus, autophagy serves as the body's cellular cleaning and recycling system.
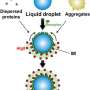
Researchers have long been studying the mechanisms related to autophagy and its role in potentially preventing and fighting diseases. p62 protein is an important part of this process. It forms a membrane-less cytosolic organelle called a p62 body and helps in the selective removal of toxic cellular waste through autophagy.
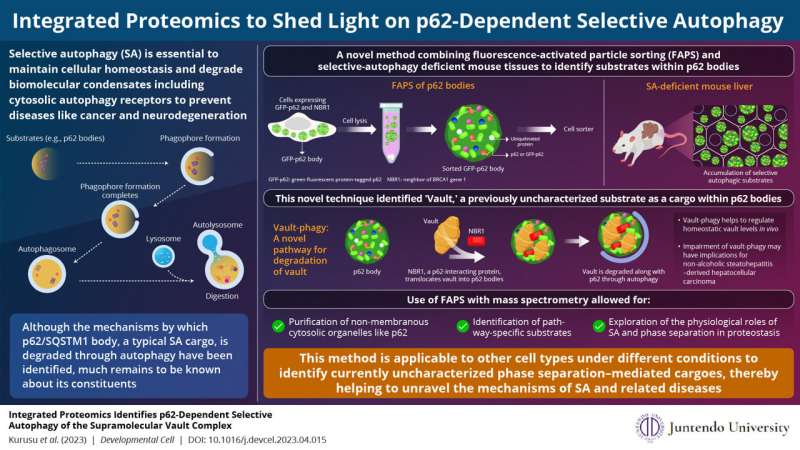
Regulated removal of unwanted proteins via selective autophagy of p62 bodies maintains cellular homeostasis. However, their compromised clearance leads to the accumulation of p62 and proteins inside p62 bodies, resulting in several diseases, such as hepatocellular carcinoma. While studies have helped us understand the mechanisms associated with the degradation of p62 bodies, our knowledge on their constituents remains incomplete.
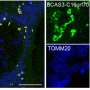
To this end, a group of scientists led by Associate Professor Hideaki Morishita and Professor Masaaki Komatsu from Juntendo University Graduate School of Medicine, Japan, have developed a novel method that uses fluorescence-activated particle sorting (FAPS) to purify p62 bodies. They followed it with mass spectrometry of the purified organelles and the tissues retrieved from selective autophagy-defective mice, to identify the substrates within p62 bodies.
Their analyses not only identified several previously known autophagy substrates and receptors present within p62 bodies, but also unraveled a major subunit of the supramolecular protein complex, vault, as a cargo within the membrane-less organelle. Their findings are published in the journal Developmental Cell on May 15, 2023.
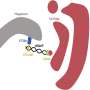
The team also included Reo Kurusu and Yuki Fujimoto, undergraduate students from the Program for Training Physicians Conducting Basic Research, Juntendo University. The scientists elaborate that vault is recruited to p62 bodies and degraded by selective autophagy, a process called vault-phagy. This is achieved through an intermediary protein called NBR1, which is critical for the employment of vault and its subsequent degradation along with p62 bodies.
Why is this finding important? According to the scientists, vault-phagy, i.e., the degradation of vault, regulates homeostatic vault levels within cells and tissues, and its impairment may be associated with non-alcoholic steatohepatitis-derived hepatocellular carcinoma, among others.
Discussing their study, Prof. Masaaki says, "Our proteomic approach identified the contents of p62 bodies in detail and enabled us to determine the substrates of p62-mediated selective autophagy. This method can be applied to other cell types and tissues under several conditions, like stress and diseases."
Although there is evidence in the literature on methods to identify selective autophagy substrates, the present method using FAPS and selective autophagy–defective mice has several advantages. FAPS is more suitable for the purification of non-membranous cytosolic organelles like p62 bodies. Furthermore, it can identify pathway-specific substrates and can be instrumental in understanding the physiological role of selective autophagy and phase separation in proteostasis.
Associate Professor Hideaki Morishita says, "Our methods have a broad scope of potential applications that should lead to the discovery of currently uncharacterized phase separation–mediated cargoes in order to allow for a deeper understanding of selective autophagy and related diseases."
More information: Masaaki Komatsu, Integrated proteomics identifies p62-dependent selective autophagy of the supramolecular vault complex, Developmental Cell (2023). DOI: 10.1016/j.devcel.2023.04.015. www.cell.com/developmental-cel … 1534-5807(23)00191-0
Journal information: Developmental Cell
Provided by Juntendo University Research Promotion Center