This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Fine particulate matter found to catalyze oxidative stress in the lungs
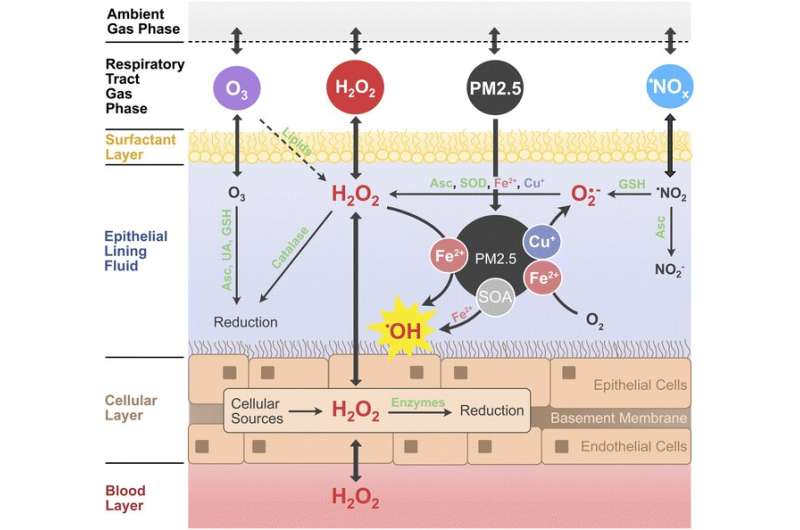
A new study conducted by a team of scientists from the Max Planck Institute for Chemistry (MPIC) reveals that the adverse health effects of fine particulate matter (PM2.5) are attributable to the conversion of peroxides into more reactive species such as the hydroxyl radical (OH) rather than the direct chemical production of hydrogen peroxide (H2O2) as previously thought.
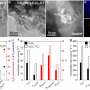
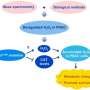
In the scientific literature, the total production of reactive oxygen species (ROS) such as H2O2 is commonly used as proxy for the toxicity of air pollutants and their ability to induce oxidative stress and inflammation. The research team led by Thomas Berkemeier from the MPIC in Mainz found that ROS concentrations in the epithelial lining fluid (ELF) of the human respiratory tract may be primarily determined by the release of endogenous H2O2 and the inhalation of ambient gas-phase H2O2, while the chemical production of H2O2 through inhaled PM2.5 is less important.
"Based on our simulations, we think that the overall concentrations of these reactive species in the lungs are large anyway, and not directly dependent on levels of air pollution," says Dr. Thomas Berkemeier, head of the Chemical Kinetics & Reaction Mechanisms group at the MPIC. They used a computer model to understand the relevant physical, chemical, and biological processes, and quantify the health effects of different types of air pollutants.
"Our new model simulates the chemical reactions that happen in the respiratory tract. For the first time, we included production, diffusion, and removal of hydrogen peroxide from cells and the blood stream into our computer model. This was quite challenging, because it is not so easy to put these processes in biological tissues into equations," explains Thomas Berkemeier.
New research directions
"The findings of this study suggest that the current paradigms for assessing the differential toxicity of individual PM2.5 components need to be critically reassessed," says Prof. Dr. Ulrich Pöschl, Head of the Multiphase Chemistry Department at the MPIC. The study proposed that the chemical production of superoxide and H2O2 in a cell-free assay may not be a suitable metric for assessing the differential toxicity of individual PM2.5 components, and some acellular oxidative potential assays may not capture the actual deleterious effects of PM2.5.
Fine particulates might act through Fenton chemistry
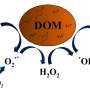
However, the production of hydroxyl radicals (OH) was strongly correlated with Fenton chemistry of PM2.5 in the model calculations. "The model simulations suggest that PM2.5 mostly acts by conversion of peroxides into highly reactive OH radicals. Thus, PM2.5 is not so much the fuel, but rather the catalyst of the chemical reactions that cause damage to cells and tissues," says Berkemeier explaining the role of inhaled particles in the model.
Additionally, PM2.5 may stimulate the production of superoxide from endogenous sources, which further contributes to the adverse health effects of air pollution.
The study underscores the importance of continued research to better understand the chemical mechanisms underlying the health effects of air pollution and to develop effective strategies to mitigate these effects. The authors believe that this study will contribute significantly to this important research effort. Their findings are published in Environmental Science: Atmospheres.
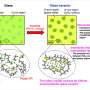
Air pollution is a major health risk that affects millions of people worldwide, but the underlying chemical mechanisms are not yet fully understood. Fine particulate matter (PM2.5) typically contains chemical components that can trigger oxidation reactions. When inhaled and deposited in the human respiratory tract, they can induce and sustain radical reaction cycles that produce reactive oxygen species (ROS) in the epithelial lining fluid (ELF) that covers the airways and alveoli in human lungs.
Numerous studies have shown that excess concentrations of ROS like hydrogen peroxide (H2O2) and hydroxyl radicals (OH) can cause oxidative stress injuring cells and tissues in the respiratory tract.
More information: Eleni Dovrou et al, Influence of ambient and endogenous H2O2 on reactive oxygen species concentrations and OH radical production in the respiratory tract, Environmental Science: Atmospheres (2023). DOI: 10.1039/D2EA00179A
Provided by Max Plank Institute for Chemistry