This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
A safe synthesis of hydrogen peroxide inspired by nature
Hydrogen peroxide is a ubiquitous chemical found in most homes and used in everything from dying hair to treating wounds. It is also an invaluable agent for many industries from food, textiles, and even in semiconductor production.
Reporting in the Journal of the American Chemical Society, researchers at Kyushu University have developed a new process to synthesize this chemical utilizing a new homogeneous catalyst inspired by nature. Moreover, the process is significantly safer than conventional methods.
Scientists have been reporting the synthesis of hydrogen peroxide for more than two hundred years. Today's industry standard is a method known as the 'anthraquinone method,' a stepwise and indirect process that dates back to World War II. However, while hydrogen peroxide—or H2O2—itself is relatively harmless, its production sometimes is not.
"Current synthesis methods are wasteful. This is a result of the high flammability of the hydrogen and oxygen mixtures and the requirement of varying materials," explains Professor Seiji Ogo from Kyushu University's Faculty of Engineering, who authored the study.
Ogo and his team have been seeking a greener approach for H2O2 production, looking to nature for inspiration. They focused particularly on a set of enzymes called 'hydrogenases.' These are enzymes found in extremophile microorganisms—organisms that live in extremely hostile environments, like the very bottom of the ocean.
Another location of extremophile activity is the hot springs of Japan. This is where Ogo and colleagues found hydrogenase S-77. Hydrogenases show promising properties for the transfer of electrons from hydrogen to oxygen molecules, a key event for the synthesis of hydrogen peroxide.
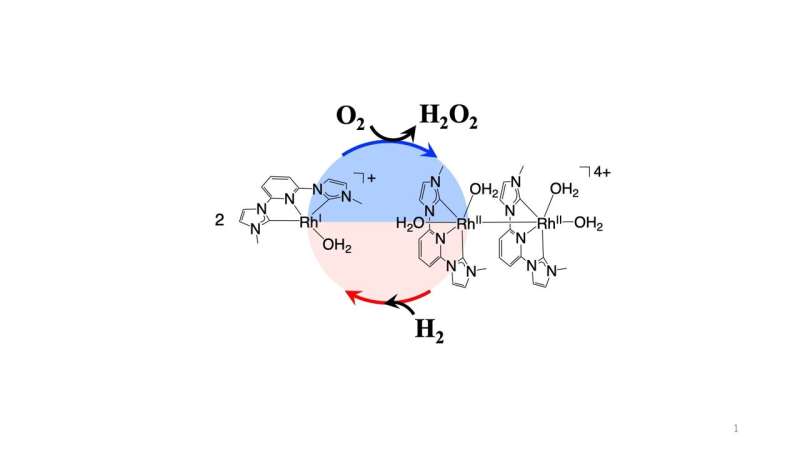
"Hydrogenase S-77 was the source of inspiration for previous work in my lab, where we constructed a rhodium-based homogeneous catalyst," explains Ogo. "The catalyst worked by facilitating the withdrawal of electrons from hydrogen molecules and then donating them to oxygen molecules. However, it gave a poor yield."
In their new study, the catalyst was improved by incorporating a N-heterocyclic carbene ligand for the reduction stage, which led to a turnover number—a reflection of the efficiency of the reaction—more than 250 times higher that of the previous catalyst, and one of the highest ever reported for a homogeneous catalyst.
Further, unlike the conventional anthraquinone method, the new homogeneous catalyst requires only one step and no separation of hydrogen and oxygen gas from the reaction flask. Therefore, with more development, the new catalyst could lead to a process that demands less material and lower costs at industrial levels.
"The new process is an ideal reaction for hydrogen peroxide synthesis because it involves a safe reaction mixture, reaction in one vessel, and direct synthesis using only hydrogen and oxygen," concludes Ogo. "Companies are always seeking ways to lower production costs. Our catalyst may be an important resource for many industries."
More information: Seiji Ogo et al, Safe, One-Pot, Homogeneous Direct Synthesis of H2O2, Journal of the American Chemical Society (2023). DOI: 10.1021/jacs.2c13149
Journal information: Journal of the American Chemical Society
Provided by Kyushu University