This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Cellular traffic controllers caught managing flow of signals from receptors

Proteins that act like air traffic controllers, managing the flow of signals in and out of human cells, have been observed for the first time with unprecedented detail using advanced microscopy techniques.
Described in new research published today (May 4) in Cell, an international team of researchers led by Professor Davide Calebiro from the University of Birmingham has seen how beta-arrestin, a protein involved in managing a common and important group of cellular gateways, known as receptors, works.
Beta-arrestin is involved in controlling the activity of G protein-coupled receptors (GPCRs) which are the largest group of receptors in the human body and mediate the effects of many hormones and neurotransmitters. As a result, GPCRs are major targets for drug development and between 30% and 40% of all current therapeutics are against these receptors. Once the receptors are activated, beta-arrestins dampen the signal in a process called desensitization but can also mediate signals of their own.
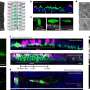
The new study published in Cell has unexpectedly revealed that beta-arrestins attach themselves to the outer cell membrane waiting for hormones or neurotransmitters to land on receptors. Surprisingly, the interactions between beta-arrestins and active receptors are much more dynamic than previously thought, allowing for a far better control of receptor-mediated signals.
Davide Calebiro, Professor of Molecular Endocrinology in the Institute of Metabolism and Systems Research at the University of Birmingham and Co-Director of the Center of Membrane Proteins and Receptors (COMPARE) of the Universities of Birmingham and Nottingham said,
"In our study, we used innovative single-molecule microscopy and computational methods developed in our lab to observe for the first time how individual beta-arrestin molecules work in our cells with unprecedented detail.
"We have revealed a new mechanism that explains how beta-arrestins can efficiently interact with receptors on the plasma membrane of a cell. Acting like air traffic controllers, these proteins sense when receptors are activated by a hormone or a neurotransmitter to modulate the flow of signals within our cells. By doing so, they play a key role in signal desensitization, a fundamental biological process that allows our organism to adapt to prolonged stimulation.
"These results are highly unexpected and could pave the way to novel therapeutic approaches for diseases such as heart failure and diabetes or the development of more effective and better tolerated analgesics."
Pioneering research methods could lead to novel drug therapies
This success was only possible thanks to the unique multidisciplinary collaborative environment provided by COMPARE, a world-leading research center for the study of membrane proteins and receptors that brings together 36 research groups with complementary expertise in cell biology, receptor pharmacology, biophysics, advanced microscopy and computer science.
The novel single-molecule microscopy and computational approaches developed in this study could provide a significant new tool for future drug development, allowing researchers to directly observe how therapeutic agents modulate receptor activity in living cells with unprecedented detail. In the future, COMPARE researchers led by Prof Calebiro plan to further automate the current pipeline so that it can be used to screen for novel drugs such as biased opioids currently in development for the treatment of pain.
Dr. Zsombor Koszegi, who shares first co-authorship of the study with Dr. Jak Grimes and Dr. Yann Lanoiselée, said, "Being able to see for the first time how individual receptors and beta-arrestins work in our cells was incredibly exciting. Our findings are highly unexpected and bring our understanding of the way beta-arrestin coordinates receptor signaling to a whole new level, with major implications for cell biology and drug discovery."
More information: Davide Calebiro, Plasma membrane preassociation drives β-arrestin coupling to receptors and activation, Cell (2023). DOI: 10.1016/j.cell.2023.04.018. www.cell.com/cell/fulltext/S0092-8674(23)00414-2
Journal information: Cell
Provided by University of Birmingham