This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
New catalyst design for electrocatalytic acetylene semihydrogenation
Recently, Prof. Zeng Jie's research group from Hefei National Research Center for Physical Sciences at the Microscale and University of Science and Technology of China (USTC), collaborating with Prof. Xia Chuan and Researcher Zheng Tingting's team from the University of Electronic Science and Technology of China, developed an undercoordinated Cu nanodots catalyst to achieve acetylene semihydrogenation with high intrinsic activity and efficiency. The research was published in Nature Communications.
Ethylene is a prominent material in chemical industry. Normally, the industrial production process of ethylene (C2H4) unavoidably produces 0.5%-3% acetylene (C2H2) byproduct that irreversibly poisons the Ziegler-Natta catalysts, resulting in a decline in activity.
Therefore, it is important to remove the C2H2 impurities in ethylene production. The traditional thermocatalytic semihydrogenation of C2H2 requires high temperature, high pressure and expensive palladium-based catalyst, which limit its further development.
The decreasing cost of electricity has motivated the development of electrocatalytic acetylene semihydrogenation (EASH) using Cu-based catalysts as an alternative. However, previous Cu-based catalysts still suffer from side reactions, which results in unsatisfactory purification.
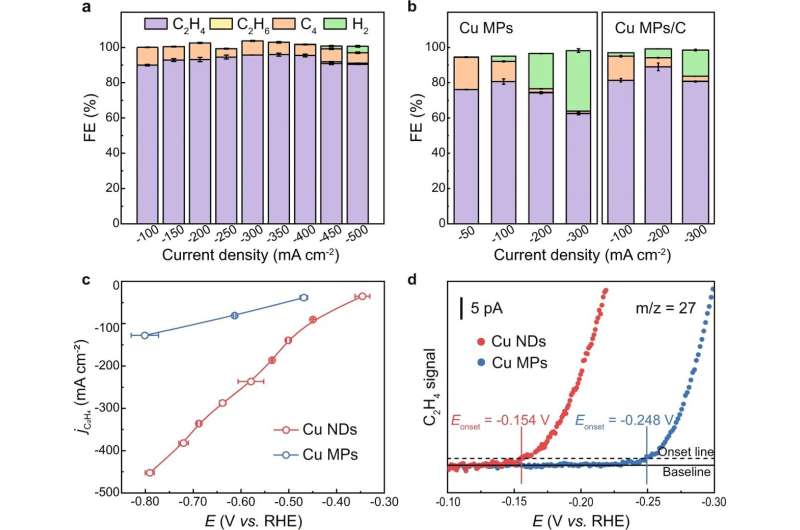
To solve this problem, the team first synthesized undercoordinated Cu nanodots (Cu NDs) as catalysts by in situ reduction. When tested for activity under pure acetylene flow, the undercoordinated Cu NDs achieved a Faradaic efficiency of over 90% under a wide range of current density, reaching a peak of 95.6% at a current density of -350mA·cm-2. Additionally, in situ spectroscopy revealed a lower energy barrier for undercoordinated Cu NDs catalysts.
Membrane electrode assembly (MEA) reactors have the advantages of low resistance, low energy consumption and compact configuration. Therefore, the team designed an MEA-type-two-electrode reactor for continuous generation of polymer-grade C2H4.
In the performance evaluation test, a 25 cm2 MEA reactor could completely convert 0.5% C2H2 to C2H4 at different flow rates from 10 to 50 standard cubic centimeter per minute (sccm). With the increase of the flow rate, the selectivity of C2H4 showed an upward trend. The team managed to continuously synthesize polymer-grade C2H4 for 130 hours under a flow rate of 50 sccm with negligible performance decay (residual C2H2 less than 1 part per million, cell voltage maintained at -1.89V).
This work realized highly efficient EASH to produce polymer-grade ethylene through catalyst development, reaction mechanism study and reactor design, providing prospects for future development of electrocatalytic ethylene purification.
More information: Weiqing Xue et al, Electrosynthesis of polymer-grade ethylene via acetylene semihydrogenation over undercoordinated Cu nanodots, Nature Communications (2023). DOI: 10.1038/s41467-023-37821-1
Journal information: Nature Communications
Provided by University of Science and Technology of China