This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Novel living yeast-based dual biosensor for detecting peptide variants
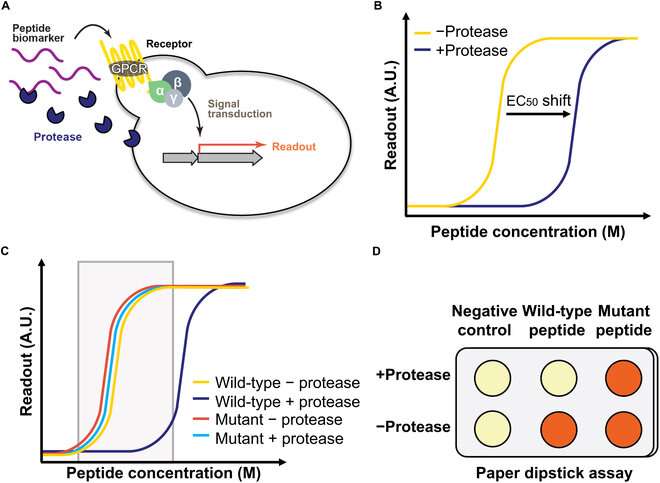
Biosensors—sensors that can detect biological samples—are powerful tools for understanding the function, composition, and structure of biochemical molecules. Biosensors are often applied for the detection of proteins and their subunits, called peptides, yielding a wide range of biomedical applications.
In 2017, researchers from Columbia University in U.S. engineered a living yeast biosensor by rewiring pheromone-related signaling pathways used by yeast for mating. In the presence of the pheromone peptide, the G-protein coupled receptor (GPCR) could detect the peptide, triggering a cascade that would eventually activate a pigment called lycopene that gives tomatoes their red color.
Thus, through a simple color change visible to the naked eye, the yeast biosensor could signal the presence of a particular peptide. However, this system lacked a peptide-cleaving catalytic enzyme called protease, the addition of which was anticipated to enhance its biosensing and discrimination abilities.
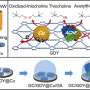
Accordingly, in a recent study published in BioDesign Research, the group developed a new and improved dual version of their living yeast biosensor by incorporating co-expressed yeast proteases.
The principal investigator of this study, Prof. Virginia W. Cornish, explains, "Our goal was to develop a dual biosensor. In the first part, the biosensor without the protease would detect the presence of all peptide variants. In the second part, the protease would be present. Only one variant of the protein would be cleaved by the protease so that a color change would be visible only for that specific variant. Here, we tried to develop a proof-of-concept for this sensing model."
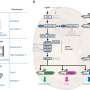
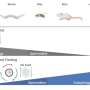
The development of this state-of-the-art biosensor was a long and technically challenging process. The researchers retained their original model, exploiting the mating pathways in yeast, and examined the dose–response curves of five fungal pheromone GPCRs, peptides, and proteases from Saccharomyces cerevisiae, Candida albicans, Schizosaccharomyces pombe, Schizosaccharomyces octosporus, and Schizosaccharomyces japonicus. Of these, the first two provided the most selective responses.
They then analyzed the peptides from these two species, i.e., S. cerevisiae and C. albicans, using alanine scanning—a technique that reveals how specific parts of a peptide contribute to its stability and function. Alanine scanning was performed with and without the protease.
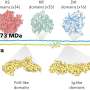
Accordingly, two peptide variants that could not be cleaved efficiently by the protease were identified: CaPep2A and CaPep2A13A. Meanwhile, their sister peptides—CaPep and CaPep13A, respectively—could be cleaved efficiently. Moreover, the color changes could be observed with the naked eye, without any need for specialized equipment.
These components were combined in a living yeast cell to develop the dual-phase biosensor. Proof-of-concept experiments revealed that the biosensor could not only detect the presence of CaPep/CaPep2A and CaPep13A/CaPep2A13A but also distinguish between them. Thus, as expected, the reintroduction of the protease enhanced the capabilities and potential applications of the original biosensor to a great extent.
According to Prof. Cornish and her team, this work is the first fundamental step towards developing a biosensor that could distinguish between a wide variety of peptides. "Synthetic biology is a step-by-step process. The framework developed in the current study can be improved through additional engineering via computational modeling and directed evolution. This will broaden the scope of biosensor's detection capabilities," she comments.
"We could use these protease-containing biosensors in point-of-care diagnostic tools and drug testing, and even to develop a scalable communication language. The possibilities are endless," she concludes, describing her vision for the future.
Overall, this study provides key insights into the manipulation of yeast mating components for developing synthetic biology tools. The findings are a testament to the exciting developments in the field of bioengineering and its potential to change our future.
More information: Tea Crnković et al, Peptide Variant Detection by a Living Yeast Biosensor via an Epitope-Selective Protease, BioDesign Research (2023). DOI: 10.34133/bdr.0003
Provided by NanJing Agricultural University