This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A new mechanism for shaping animal tissues
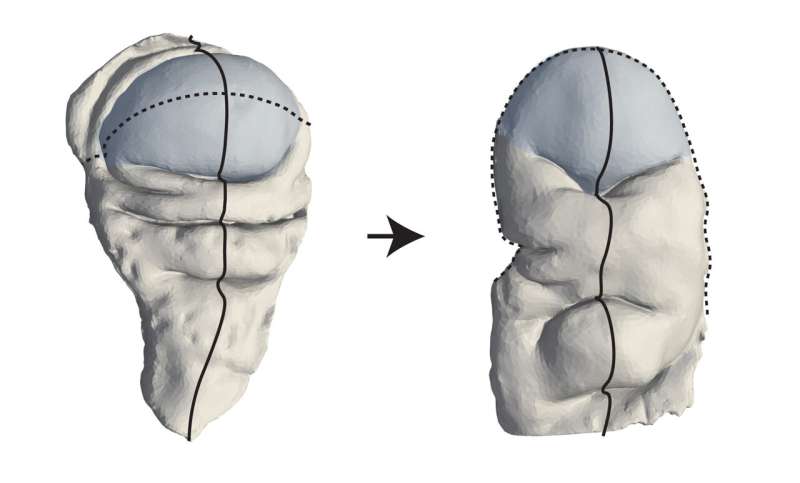
A key question that remains in biology and biophysics is how three-dimensional tissue shapes emerge during animal development. Research teams from the Max Planck Institute of Molecular Cell Biology and Genetics (MPI-CBG) in Dresden, Germany, the Excellence Cluster Physics of Life (PoL) at the TU Dresden, and the Center for Systems Biology Dresden (CSBD) have now found a mechanism by which tissues can be "programmed" to transition from a flat state to a three-dimensional shape.
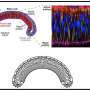
To accomplish this, the researchers looked at the development of the fruit fly Drosophila and its wing disk pouch, which transitions from a shallow dome shape to a curved fold and later becomes the wing of an adult fly. The researchers developed a method to measure three-dimensional shape changes and analyze how cells behave during this process. Using a physical model based on shape-programming, they found that the movements and rearrangements of cells play a key role in shaping the tissue.
This study, published in Science Advances, shows that the shape programming method could be a common way to show how tissues form in animals.
Epithelial tissues are layers of tightly connected cells and make up the basic structure of many organs. To create functional organs, tissues change their shape in three dimensions. While some mechanisms for three-dimensional shapes have been explored, they are not sufficient to explain the diversity of animal tissue forms.
For example, during a process in the development of a fruit fly called wing disk eversion, the wing transitions from a single layer of cells to a double layer. How the wing disk pouch undergoes this shape change from a radially symmetric dome into a curved fold shape is unknown.
The research groups of Carl Modes, group leader at the MPI-CBG and the CSBD, and Natalie Dye, group leader at PoL and previously affiliated with MPI-CBG, wanted to find out how this shape change occurs.
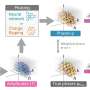
"To explain this process, we drew inspiration from 'shape-programmable' inanimate material sheets, such as thin hydrogels, that can transform into three-dimensional shapes through internal stresses when stimulated," explains Dye.
"These materials can change their internal structure across the sheet in a controlled way to create specific three-dimensional shapes. This concept has already helped us understand how plants grow. Animal tissues, however, are more dynamic, with cells that change shape, size, and position."
To see if shape programming could be a mechanism to understand animal development, the researchers measured tissue shape changes and cell behaviors during the Drosophila wing disk eversion, when the dome shape transforms into a curved fold shape.
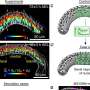
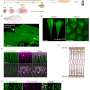
"Using a physical model, we showed that collective, programmed cell behaviors are sufficient to create the shape changes seen in the wing disk pouch. This means that external forces from surrounding tissues are not needed, and cell rearrangements are the main driver of pouch shape change," says Jana Fuhrmann, a postdoctoral fellow in the research group of Dye.
To confirm that rearranged cells are the main reason for pouch eversion, the researchers tested this by reducing cell movement, which in turn caused problems with the tissue shaping process.
Abhijeet Krishna, a doctoral student in the group of Modes at the time of the study, explains, "The new models for shape programmability that we developed are connected to different types of cell behaviors. These models include both uniform and direction-dependent effects. While there were previous models for shape programmability, they only looked at one type of effect at a time. Our models combine both types of effects and link them directly to cell behaviors."
Dye and Modes conclude, "We discovered that internal stress brought on by active cell behaviors is what shapes the Drosophila wing disk pouch during eversion. Using our new method and a theoretical framework derived from shape-programmable materials, we were able to measure cell patterns on any tissue surface. These tools help us understand how animal tissue transforms their shape and size in three dimensions.
"Overall, our work suggests that early mechanical signals help organize how cells behave, which later leads to changes in tissue shape. Our work illustrates principles that could be used more widely to better understand other tissue-shaping processes."
More information: Jana Fuhrmann et al, Active shape programming drives Drosophila wing disc eversion, Science Advances (2024). DOI: 10.1126/sciadv.adp0860, www.science.org/doi/10.1126/sciadv.adp0860
Journal information: Science Advances
Provided by Max Planck Society