This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
How a mutation in the SKD3 enzyme can cause MGCA7 disease
Researchers at Baylor College of Medicine and collaborating institutions report in the journal Nature Communications how a mutation in the enzyme SKD3 can cause a form of a genetic disease known as 3-methylglutaconic aciduria (MGCA7). MGCA7 is an inborn error of metabolism associated with variable neurologic deficits and an abnormally low number of immune cells called neutrophils in the blood.
The latter condition, known as neutropenia, can lead to increased susceptibility to infection and can also develop into leukemia, as well as early death in infants.
"SKD3 is essential to protein quality control in animal cells. It removes damaged proteins in structures or organelles inside cells called mitochondria thus maintaining the integrity of these organelles, which is vital for normal cell function," said corresponding author Dr. Francis Tsai, professor of biochemistry and molecular biology, molecular and cellular biology and molecular virology and microbiology at Baylor.
"Failure of the protein quality control machinery to clear misfolded proteins, for instance, results in the formation of protein aggregates and toxic forms of defective proteins, which are hallmarks of many human diseases."
SKD3 belongs to a family of proteins called unfoldases, which are widely found in microbes. Here, Tsai and his colleagues focused on the unfoldase that is present in humans and whose mutations cause MGCA7.
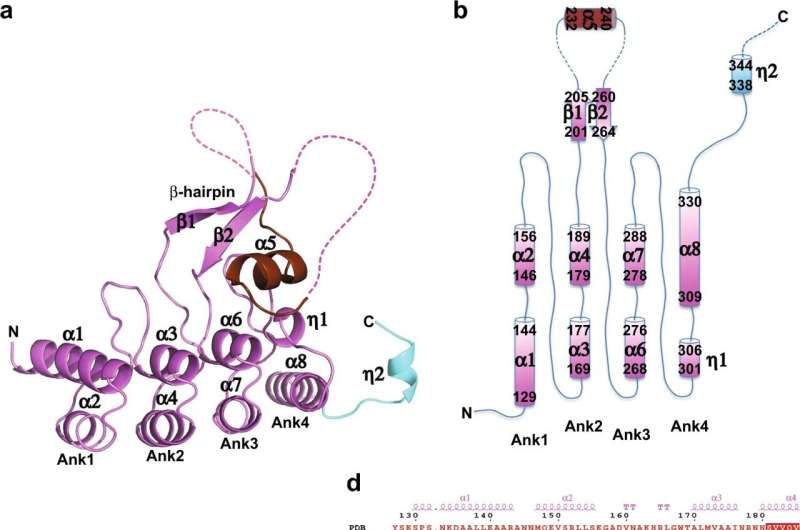
SKD3 enzymes have a catalytic domain or part that drives protein unfolding, and a non-catalytic domain of unknown function. "Previous studies have shown that mutations in the catalytic domain that disrupt SKD3 activity can cause MGCA7 disease, but it's been a mystery how mutations in the non-catalytic domain would lead to the disease. That is what we focused on in this study," Tsai said.
Imagine enzyme SKD3 is like a car. A car has an engine (i.e., catalytic domain) that moves the car forward and requires fuel (i.e., ATP) to do so. A malfunctioning engine (i.e., a mutation in the catalytic domain) will prevent the car from moving. However, a car also needs tires (i.e., non-catalytic domains). An engine malfunctioning or a tire puncture will bring the car to a stop.
The researchers discovered that one mutation in the enzyme's non-catalytic domain leads to the formation of a bond that staples parts of the non-catalytic domain together. This would result in a change in the 3D structure that inactivates the enzyme.
Experimentally, the team confirmed that this mutant enzyme causes massive protein aggregation in cells where the enzyme function is crucial for maintaining the structure of mitochondria. Without this specific mutation in the non-catalytic domain, the enzyme retains its normal function. The next step would be to determine what is the 3D conformation in mutant SKD3 that causes MGCA7 disease.
"This work provides a new understanding of mitochondrial biology. Mitochondria generate ATP, the primary energy source of all living cells," Tsai said. "We have provided evidence that SKD3 is a central player in maintaining protein quality control in mitochondria and have proposed the first mechanism by which non-catalytic domain mutations in SKD3 can lead to MGCA7."
More information: Sukyeong Lee et al, Structural basis of impaired disaggregase function in the oxidation-sensitive SKD3 mutant causing 3-methylglutaconic aciduria, Nature Communications (2023). DOI: 10.1038/s41467-023-37657-9
Journal information: Nature Communications
Provided by Baylor College of Medicine