This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Understanding how cancer cells migrate paves the way for targeted therapies
Like deactivating a bomb by cutting the correct wire, what if we can disable a cancer cell from spreading by "terminating" the right switch in its machinery? A team of researchers from the Mechanobiology Institute and the Department of Biological Sciences, National University of Singapore, with their local and overseas collaborators, might have identified that key component—a scaffold protein known as BPGAP1, and how it works. Their research has been featured in the journal Molecular Biology of the Cell in its "Forces On and Within Cells" Special Issue.
Led by Dr. Darren Wong with the guidance of Assoc. Prof. Low Boon Chuan, the team discovered how BPGAP1 synchronizes two key proteins responsible for cell migration—namely, GTPases Rac1 and RhoA, which Dr. Wong described as "the two hands which work hand-in-hand to move the cell." The migrative ability of the cell is what enables metastasis, which is when cancer cells depart from their original site, travel through our bloodstreams, and invade distant organs; It is also what makes cancer so devastatingly deadly.
Being a complicated and multistep process, effective treatment options for metastasis are limited and cater more towards symptom relief at advanced stages than eliminating root causes. Unraveling the underlying mechanistic action of BPGAP1 may be the key to apprehending the rouge traversal of cancer cells and paving the way to create more directed approaches to cancer intervention.
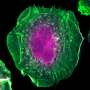
The mobility of a cell is propelled by changes in its cytoskeletal organization, which in turn is governed by a set of proteins, including a group known as GTPases. GTPase can be described as a molecular switch which activates (or inactivates) specific pathways to carry out cellular functions. In this case, GTPases Rac1 and RhoA work with each other to remodel the cytoskeleton by mediating different pathways—Rac1 enables the cell to sense, grip onto its surroundings and crawl by forming sheet-like membrane protrusions (known as lamellipodia), while RhoA generates adhesion sites and contractile force to power the cell forward. These two processes typically antagonize each other and do not take place in the same place and time, but are needed to ensure effective cell movements. Hence, there is a need to orchestrate the proteins in a way that their functions are in sync.
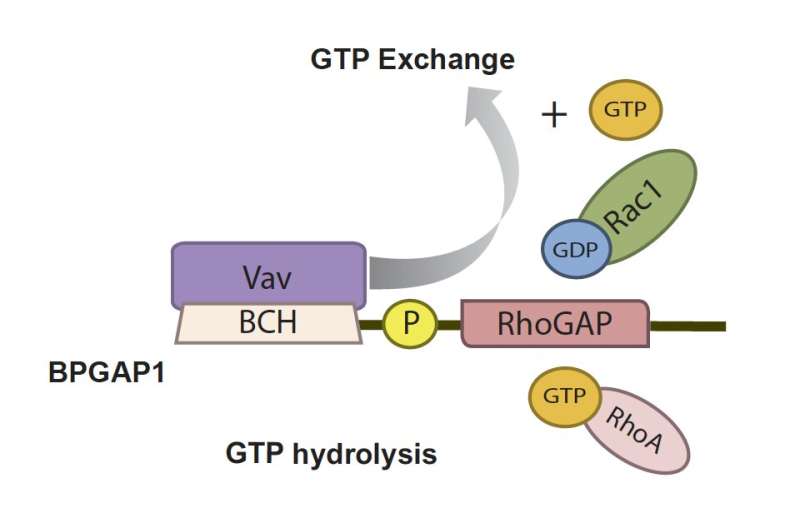
While scientists recognize Rac1 and RhoA's involvement in cell movement, Dr. Wong and his team found that another protein, BPGAP1, does not only have interactions with both of them, but is also highly expressed in cancer cells and extensively promotes cell migration. They later revealed that BPGAP1 acts as a scaffold and coordinator between the two GTPases, therefore serving as a key regulator of their activities.
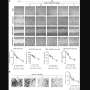
To validate this, they collaborated with clinical scientists locally and overseas. By using various models, assays and biomaterials, and mostly manipulating metastatic breast cancer cells, they managed to uncover and piece together the working mechanism of BPGAP1.
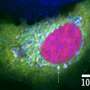
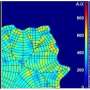
They found that BPGAP1 binds to an inactive Rac1, and, together, they relocate to the lamellipodia. To activate Rac1, BPGAP1 has to recruit another protein known as Vav1 while the cell is physiologically stimulated by epidermal growth factors. When all these factors are in place, the team spotted enhanced cell migration behaviors, such as the cell flattening out and spreading, growing longer protrusions, having a boost in mobility and a better ability to extrude itself from blood vessels.
But how does RhoA fit into all these? As opposed to Rac1, BPGAP1 inactivates RhoA by binding one of its domains to the latter. Thus, BPGAP1 coordinates the activities between the two GTPases by dually "switching off" RhoA while "switching on" Rac1. These repeated cycles of on/off switching ultimately reinforce the cell's mobility.
It is also vital, timewise, as it serves as a pacemaker to harmonize the timing of Rac1 activation with that of RhoA inactivation. Such intimate dynamics enable a rapid response to any disturbances to the cell. So unsurprisingly, the team validated that if BPGAP1 loses its function and is unable to regulate the two GTPases, it impairs the cell's ability to migrate.
By distinguishing BPGAP1's role in controlling cell movement, coupled with its greater presence in metastatic cells, it is clear to see how BPGAP1 is the epicenter of cell migration and metastasis. Moreover, being upregulated across all stages of breast cancer, as well as in cancers of the lungs, pancreas, cervix, colon, ovary and stomach, implies that it plays a part in different types and stages of cancer too.
Hence, the novel discovery of the BPGAP1 presents immense potential for cancer prediction and mediation. "We can use BPGAP1 as both a marker for cancer prognosis and a target for cancer intervention across different cancer types," said Dr. Wong, "We hope that with this breakthrough, we can inspire new approaches for therapeutic designs pertaining to cancer and metastasis."
More information: Darren Chen Pei Wong et al, The scaffold RhoGAP protein ARHGAP8/BPGAP1 synchronizes Rac and Rho signaling to facilitate cell migration, Molecular Biology of the Cell (2023). DOI: 10.1091/mbc.E21-03-0099
Journal information: Molecular Biology of the Cell
Provided by National University of Singapore