This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Biologists reveal a scissor enzyme that cuts the chromatin bridge and prevents DNA damage and autoimmunity
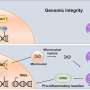
A research team led by Dr. Gary Ying Wai Chan from the School of Biological Sciences at The University of Hong Kong (HKU), has revealed the function of a unique enzyme, ANKLE1. ANKLE1 acts on chromatin bridges that are trapped in the midzone of the dividing cells. By cutting these bridges, ANKLE1 prevents damage to the genetic material and stops the immune system from mistakenly attacking the body's own cells.
Understanding the mechanism by which chromatin bridges are cleaved during cell division is important for developing new strategies to prevent or treat diseases such as cancer and autoinflammatory disorders. The research has recently been published in Advanced Science.
During cell division, the DNA must be divided properly into the two new cells. However, errors can occur during this process, causing parts of the DNA to stick together and form structures called chromatin bridges. These bridges are like strings of DNA that connect two segregating masses of chromosomes in the new cell. As a result, they would inevitably be trapped in the middle of the cell division process.
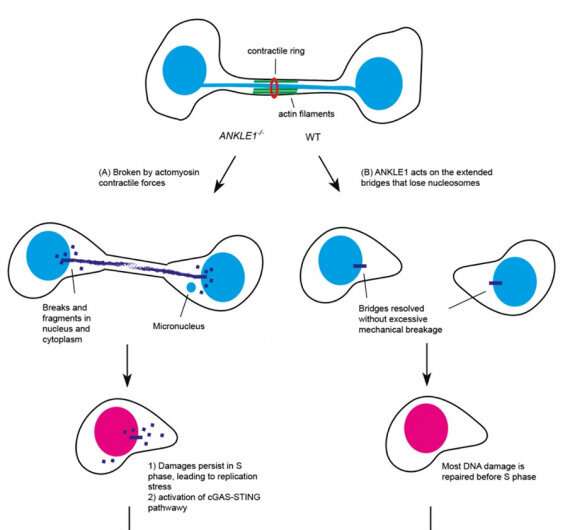
When chromatin bridges are trapped in the midzone, they will be broken by contractile forces mediated by the actin-myosin complex, which can lead to DNA damages and the formation of small nuclei called "micronuclei," causing genome instability, a characteristic of many solid tumors and activating innate immune responses that contribute to autoinflammation.
To investigate the alternative mechanism that cells employ to resolve chromatin bridges, the research team has recently discovered that ANKLE1, a unique endonuclease (enzyme), plays a crucial role in cleaving chromatin bridges at midbody during cell division, thus avoiding catastrophic breakage caused by mechanical forces. This alternative mechanism helps ensure proper DNA segregation and stability, which is important for preventing diseases such as cancer and autoinflammatory disorders.
The role of ANKLE1 in preventing DNA damage and autoimmunity
Previously, scientists identified an endonuclease called LEM-3 in Caenorhabditis elegans, a microscopic roundworm commonly used as a model organism in biological research. LEM-3 has been found to play a role in resolving chromatin bridges at the "midbody," a structure that connects the two daughter cells during cell division. The midbody is eventually severed, a process known as abscission, which results in the complete separation of the two cells.
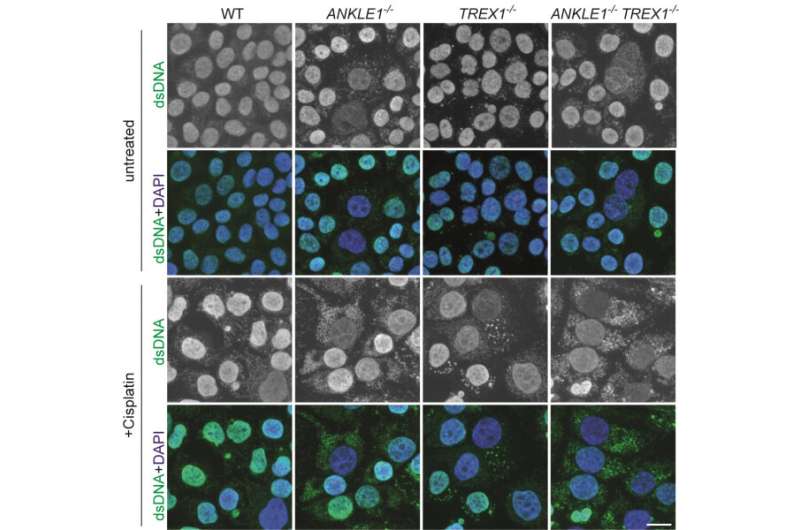
To understand the cellular functions of ANKLE1, which is the human equivalent of LEM-3, the research team employed the CRISPR/Cas9 genome editing technology to delete the ANKLE1 gene in human cells. The team found that the loss of ANKLE1 leads to increased formation of extended chromatin bridges, suggesting that cleavage of bridges by ANKLE1 prevents further stretching of the DNA. In the absence of ANKLE1, the persistent bridges would be eventually broken by the actomyosin contractile forces that separate the two daughter cells. This mechanical breakage induces massive fragmentation of DNA, resulting in the formation of micronuclei and DNA released into the cytosolic DNA, which can trigger immune responses and lead to inflammation.
Importantly, loss of ANKLE1 not only induces DNA damage and genome instability, but also leads to a strong activation of cGAS-STING innate immunity. cGAS-STING pathway is a major innate immune defense system against pathogens by sensing the DNA present in cytosol. In the absence of ANKLE1, DNA fragments are readily generated upon bridge breakage, and the resulting cytosolic DNA is mistaken to be pathogenic DNA. These results indicate that ANKLE1 plays roles in both maintaining genome stability and preventing autoimmunity.
Research significance
The research not only has made a key discovery of a mechanism utilizing a midbody-tethered endonuclease (ANKLE1) as a "scissor" to cut chromatin bridges to prevent genome instability, but also enhances our understanding about the link between chromatin bridges and innate immune responses.
"More and more research data suggest that immune responses play important roles in determining the clinical outcomes of many of the traditional anti-cancer drugs," said Dr. Gary Ying Wai Chan. "For example, the use of antimitotic drugs is a common strategy to treat solid tumors. However, whether the induction of immune responses due to the resulting chromatin bridges is partly responsible for the antitumor effect remains unclear. The link between chromosomal instability and innate immunity will definitely be an area of cancer research with tremendous promise for breakthroughs."
More information: Huadong Jiang et al, Human Endonuclease ANKLE1 Localizes at the Midbody and Processes Chromatin Bridges to Prevent DNA Damage and cGAS‐STING Activation, Advanced Science (2023). DOI: 10.1002/advs.202204388
Journal information: Advanced Science
Provided by The University of Hong Kong