This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Tsunami in a water glass: Observing the actions of the hydrated electron
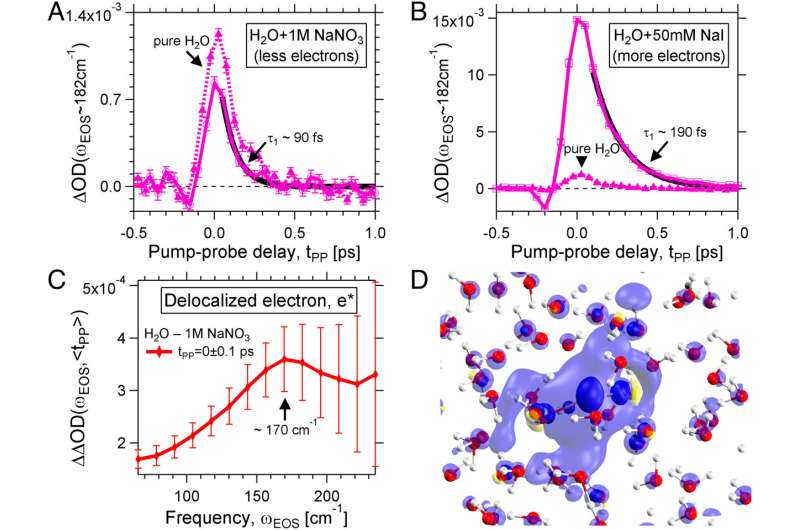
Hydrated electrons play a major role in many physical, chemical and biological processes. They are not bound to an atom or molecule and are free in the solution. Since they are only ever created as an intermediate product, they are extremely short-lived.
The team from the Cluster of Excellence Ruhr Explores Solvation RESOLV at Ruhr University Bochum was able to observe for the first time in a novel experiment how the hydrated electron affects the solution during its lifetime. The researchers led by Professor Martina Havenith-Newen report in the journal Proceedings of the National Academy of Science.
The simplest anion
"As the simplest anion, hydrated electrons represent a model system that is relevant in a multitude of radical chemical processes," says Martina Havenith-Newen. "For example, it plays an important role in energy transfer during photo- and electrochemical phenomena, in atmospheric chemistry, in radiation damage of biological substances and in medical therapy." This has earned the hydrated electron the ongoing attention of experimental and theoretical groups for several decades.
RESOLV researchers have set up a novel experiment to follow the formation and temporal evolution of the hydrated electron from the perspective of the solvent: "Immediately after its generation by means of an intense laser beam, we were able to observe a delocalized electron," Martina Havenith-Newen says.
The charge distribution extends over 20 angstroms. Within 500 femtoseconds, the charge is localized and a surprisingly stable localized electron emerges, whose fingerprint in the water network the researchers were able to observe for the first time due to the sensitivity of the experiment in the terahertz range.
"In addition, we could observe a water quake or a tsunami," says Martina Havenith-Newen. The team was able to demonstrate that this phenomenon is caused by the sudden charge separation during the formation of the hydrated electron. In contrast to atomic, negatively charged ions, the water network in the immediate vicinity is looser and not more stable. This means that the individual water molecules in the immediate vicinity of the electron can move more freely than in the water.
"This smallest anion therefore takes on a special role," sums up Martina Havenith-Newen.
More information: Fabio Novelli et al, The birth and evolution of solvated electrons in the water, Proceedings of the National Academy of Sciences (2023). DOI: 10.1073/pnas.2216480120
Journal information: Proceedings of the National Academy of Sciences
Provided by Ruhr-Universitaet-Bochum



















