How two water molecules dance together

An international research team has gained new insights into how water molecules interact. A laser with especially high brightness, as is available at the FELIX laboratory at Radboud University, was needed for the experiments. Their findings help to better better understand the strange properties of water and are published in Angewandte Chemie.
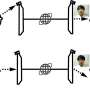
Although water is omnipresent, the interaction between individual water molecules is not yet fully understood. For the first time, the researchers were able to completely observe all of the movements between the water molecules, known as intermolecular vibrations. A certain movement of individual water molecules against each other, called hindered rotations, is particularly important.
Unknown interactions
Water is the most important solvent in chemistry and biology and possesses an array of strange properties—for instance, it reaches its highest density at four degrees Celsius. This is due to the special interactions between the water molecules. "Describing these interactions has posed a challenge for research for decades," says Martina Havenith from Ruhr-Universität Bochum.
Experiments at extremely low temperatures
The team investigated the simplest conceivable interaction, namely between precisely two individual water molecules, using terahertz spectroscopy. The researchers send short pulses of radiation in the terahertz range through the sample, which absorbs part of the radiation. The absorption pattern reveals information about the attractive interactions between the molecules. A laser with especially high brightness, as is available at Radboud University's laboratory FELIX, was needed for the experiments.
The researchers analysed the water molecules at extremely low temperatures. To do this, they successively stored individual water molecules in a tiny droplet of superfluid helium, which is as cold as 0.4 Kelvin (or -272.75 degrees Celsius). The droplets work like a vacuum cleaner that captures individual water molecules. Due to the low temperature, a stable bond occurs between two water molecules, which would not be stable at room temperature.
This experimental setup allowed the group to record a spectrum of the hindered rotations of two water molecules for the first time. "Water molecules are moving constantly," explains Martina Havenith. "They rotate, open and close." However, a water molecule that has a second water molecule in its vicinity cannot rotate freely—this is why it is referred to as a hindered rotation.
A multidimensional energy map
The interaction of the water molecules can also be represented in the form of what is known as water potential. "This is a kind of multidimensional map that notes how the energy of the water molecules changes when the distances or angles between the molecules change," explains Martina Havenith. All the properties, such as density, conductivity or evaporation temperature, can be derived from the water potential. "Our measurements now allow the best possible test of all potentials developed to date," says the researcher.
More information: Martina Havenith-Newen et al. Observation of the low frequency spectrum of water dimer as a sensitive test of the water dimer potential and dipole moment surfaces, Angewandte Chemie International Edition (2019). DOI: 10.1002/anie.201906048
Journal information: Angewandte Chemie International Edition , Angewandte Chemie
Provided by Ruhr-Universität Bochum