This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New methodology for anti-Markovnikow products
Tobias Ritter and his team have developed a methodology that can be used to form anti-Markovnikov products. This might be helpful to tackle synthesis problems. Their work has now been published in Nature Catalysis.
Vladimir Vasilyevich Markovnikov was a 19th century Russian chemist who worked at the universities of St. Petersburg, Odessa and Moscow, among others. The so-called Markovnikov rule, well known already with young students of chemistry, is named after him.
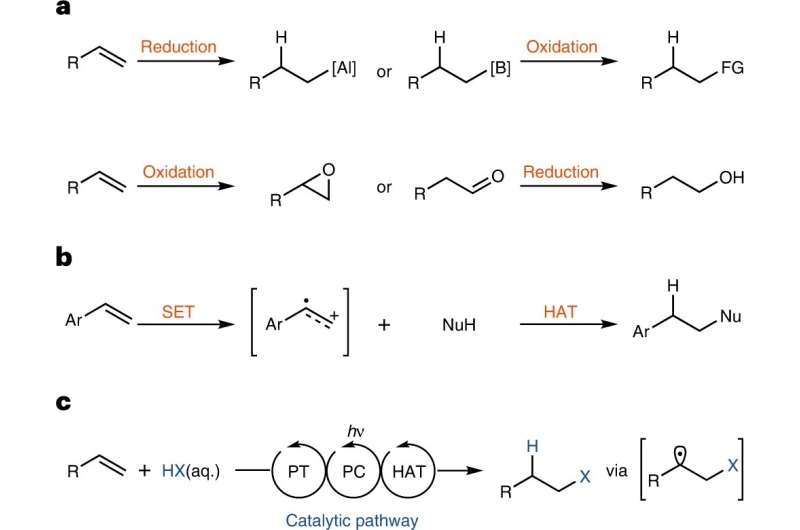
This rule says that when protic acids are added to asymmetric substituted alkenes, the hydrogen atom is attached to the carbon with more hydrogen substituents: " To him who has shall be given." Hydrochlorination of alkenes with hydrochloric acid therefore always gives the branched product, the so-called Markovnikov product.
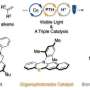
In their new paper, Prof. Tobias Ritter and his team have developed a methodology that can be used to form exactly the other, linear product—the anti-Markovnikov product. With this methodology, the group of the Max-Planck-Institut für Kohlenforschung has been able to gain new insights into the chemical behavior of molecules and thus expand the "toolbox" for chemical synthesis.
To be more specific, the chemists from Mülheim describe how ordinary hydrochloric acid can be added to alkenes with the aid of a catalyst and light, and without the need to use other substances stoichiometrically. Stoichiometry refers to the exact ratio of the amounts of the reactants needed to carry out the reaction. This is important because for each reaction scientists try to use chemicals as efficient as possible.
A certain type of catalyst plays a major role in this reaction—that means a "molecular tool" that accelerates chemical reactions or gets them running in the first place, without itself being consumed. In this case, photoredox-active ion pairs function as catalysts. These light-sensitive, electrically charged molecules are being formed during the reaction and then act as catalyst.
Although the linear products are readily accessible with other methods, the mechanism of Tobias Ritter's team may be useful to tackle further synthesis problems. Currently, anti-Markovnikov products are made by first reducing alkenes stoichiometrically and then oxidizing these products stoichiometrically. This produces a lot of waste.
"Our method allows the production of anti-Markownikov products in a way where only alkenes and hydrochloric acid are used stoichiometrically, all other components, except light, are used only as catalysts," Tobias Ritter explains. This could be useful, for example, for syntheses of primary alcohols, which are produced on a large scale using alkenes. These primary alcohols are needed in fuels and various household products.
More information: Jungwon Kim et al, Anti-Markovnikov hydrochlorination and hydronitrooxylation of α-olefins via visible-light photocatalysis, Nature Catalysis (2023). DOI: 10.1038/s41929-023-00914-7
Journal information: Nature Catalysis
Provided by Max Planck Society