This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Facile and scalable production of a fuel-cell nanocatalyst for the hydrogen economy
A fuel cell is an electric power generator that is capable of producing electricity from hydrogen gas while discharging only water as a waste product. It is hoped that this highly efficient clean energy system will play a key role in the adoption of the hydrogen economy, replacing the combustion engines and batteries in automobiles and trucks, as well as power plants.
However, the cost of platinum, which can be up to ~30,000 USD per kg, has been a major limitation, making fuel cell catalysts prohibitively expensive. The production methods of highly-performing catalysts have also been complicated and largely limited. Accordingly, the development of a facile and scalable production method for platinum-based fuel cell catalysts is an urgent challenge, together with enhancing catalytic performance and stability while using a minimum amount of platinum.
To tackle this issue, a research team led by Prof. Sung Yung-Eun and Prof. Hyeon Taeghwan at the Center for Nanoparticle Research (CNR) within the Institute for Basic Science (IBS), South Korea has discovered a novel method for the production of nanocatalysts.
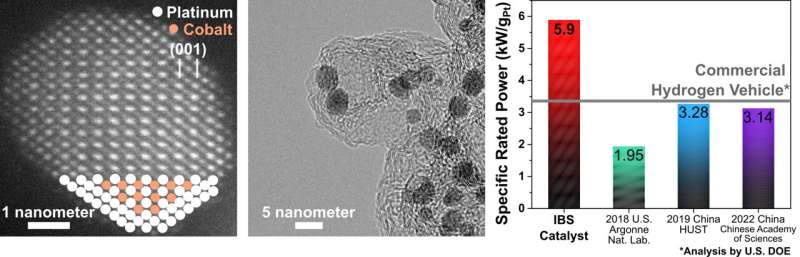
The researchers demonstrated that these uniformly sized (3-4 nanometers) cobalt-platinum (Co-Pt) alloy nanoparticles can be produced by simple heat treatment. This method has combined features of the ease of the synthesis of the impregnation method, along with the precise control over the size and shape of the nanocrystals similar to the colloidal method.
The novel Co-Pt alloy nanocatalysts developed by the CNR-IBS team consist of two oppositely-charged metal complexes, specifically Co and Pt ions surrounded by bipyridine and chlorine ligands, respectively.
The research team hypothesized that a simple heat treatment would cause the bipyridine ligand to thermally decompose into a carbon shell that can protect the growing Co-Pt alloy nanoparticles. After optimizing the heat treatment condition, they succeeded in obtaining a highly uniform nanocatalyst with nanoparticles of only 3-4 nanometer sizes.
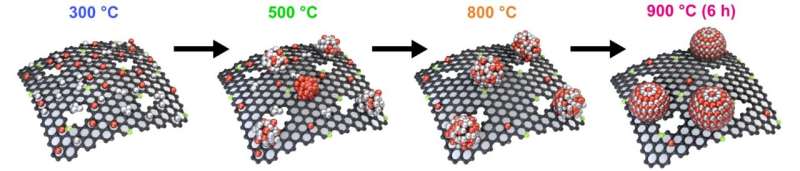
In the nanocatalyst developed by the group, Co and Pt atoms were arranged in a regular way called the 'intermetallic phase', where the unstable Co atoms are stabilized by the surrounding Pt atoms. Additionally, when nitrogen was effectively doped onto the carbon support, ionomers (proton conductors) were homogeneously dispersed over the entire catalyst layer in the fuel cell, which better facilitated the supply of oxygen gas to the surface of the Co-Pt nanocatalyst.
These structural features added up to a much-enhanced power performance in the proton-exchange-membrane fuel cell, exhibiting high specific rated power of 5.9 kW/gPt, which is about twice that of the current performance in a commercial hydrogen vehicle. The catalyst produced by the team has achieved most of the 2025 targets set by the U.S. Department of Energy (DOE) with the goal of stable long-term operation of the fuel cell.
The CNR-IBS team strongly believes that this study would stimulate the development of next-generation fuel cell catalysts. These findings would also contribute to the improvements in the catalytic performance and durability of alloy nanocatalysts for various other electrocatalytic applications.
Prof. Hyeon said, "Design of a novel bimetallic compound as a precursor material has been the critical starting point in this study. We have developed a platform technology to produce a complicated form of alloy nanocatalysts through a simple and scalable method, and finally achieved an enhanced fuel cell power performance with less amount of platinum used."
Prof. Sung said, "A world-class level of fuel cell performance has been achieved in this research, surpassing most of the 2025 targets of U.S. DOE by lessening the amount of platinum that can contribute up to around 40% of the cost of fuel cells." He added, "We expect that this study, together with some follow-up studies, would greatly impact the growth of the hydrogen vehicle industry and the realization of hydrogen economy in the near future."
The study is published in the journal Energy & Environmental Science.
More information: Tae Yong Yoo et al, Scalable production of an intermetallic Pt–Co electrocatalyst for high-power proton-exchange-membrane fuel cells, Energy & Environmental Science (2023). DOI: 10.1039/D2EE04211H
Journal information: Energy & Environmental Science
Provided by Institute for Basic Science