Researchers develop highly CO-tolerant fuel cell anode catalyst
In a study published in Angewandte Chemie International Edition., a research team, led by Prof. Gao Minrui and Prof. Yang Qing from the University of Science and Technology of China (USTC), developed a new catalyst with excellent CO-tolerance and a low cost, realizing the improved performance of fuel cells.
Given their features of high specific energy and zero emissions, hydrogen-oxygen fuel cells play an important role in achieving carbon peaking and carbon neutrality goals in China. The commercially adopted platinum on carbon (Pt/C) catalyst in full cells, however, is vulnerable to carbon monoxide (CO) poisoning. In particular, the poisoning, coupled with the slow rate of hydrogen oxidation reaction (HOR) in anion-exchange membrane fuel cells (AEMFCs), greatly worsens the cells' performance.
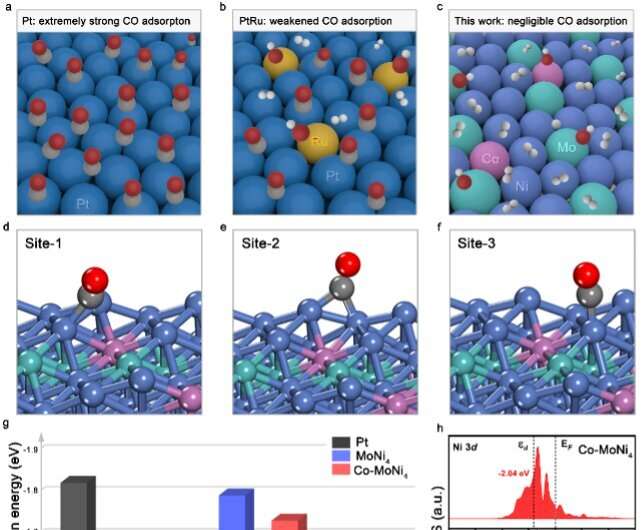
In their theoretical calculation, researchers found that the CO adsorption energy at nickel site was significantly reduced when cobalt was introduced into molybdenum-nickel alloy (MoNi4).
To solve the problems, researchers incorporated cobalt (Co) into molybdenum-nickel alloy (MoNi4) to create the Co-MoNi4 catalyst, finding that it demonstrated not only superb HOR activity in alkali, but also high CO tolerance because the incorporation of Co brings electron deficient nickel sites that lead to less d→CO 2π* back-donation, thus the weakened CO binding.
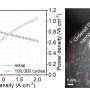
In the rotating disk electrode test, the Co-MoNi4's activity only decayed a little after 10,000 cycles in the presence of 500 parts per million (ppm) CO. The further test of the performance of AEMFCs, equipped with the catalyst, revealed a peak power density of 394 mW cm-2 in the presence of 250 ppm CO, exceeding the 209 mW cm-2 of the Pt/C catalyst, while in pure H2 the number reached 525 mW cm-2.
The study has not only made possible the curbing of CO poisoning in AEMFCs but also shed some light on creating other non-noble metal catalysts for more efficient fuel-cell applications.
More information: Yu Yang et al, Suppressing Electron Back‐Donation for a Highly CO‐tolerant Fuel Cell Anode Catalyst via Cobalt Modulation, Angewandte Chemie International Edition (2022). DOI: 10.1002/anie.202208040
Journal information: Angewandte Chemie International Edition
Provided by University of Science and Technology of China