Materials scientists create stronger cobalt for fuel cells

A multi-institutional research team led by materials scientists from Pacific Northwest National Laboratory (PNNL) has designed a highly active and durable catalyst that doesn't rely on costly platinum to spur the necessary chemical reaction.
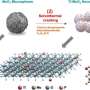
The new catalyst contains cobalt interspersed with nitrogen and carbon. When compared to a similarly structured catalyst made from iron—another promising, well-studied platinum substitute, the team found that the cobalt catalyst achieved a similar reaction but with four times the durability.
The team's research, which shows promise for fuel cells in transportation, was published in the November 30, 2020 issue of Nature Catalysis.
Seeking a replacement for costly platinum
Proton exchange membrane—or PEM—fuel cells are typically envisioned to be paired with hydrogen for multiple applications across different sectors, including transportation, stationary and backup power, metals manufacturing, and more. These highly efficient, clean energy conversion devices require very active catalysts for the chemical reaction—the oxygen reduction reaction, or the "lifeblood" that makes a fuel cell efficiently function.
Platinum group metals serve as the most productive catalyst material for PEM fuel cells, but they account for about half of the fuel cell cost.
So scientists are studying transition metals such as iron as a promising alternative to platinum, but they have found that they quickly degrade in the acidic PEM fuel cell environment.
Enter cobalt, a transition metal that is—relative to platinum—inexpensive and abundant. Previous studies had shown that cobalt is far less active than iron-based catalysts.
"We knew that the configuration of cobalt with nitrogen and carbon was key to how effectively the catalyst reacts and that the active site density was critically important for performance," said PNNL materials scientist Yuyan Shao, who led the study. "Our goal was to really improve the reaction activity of cobalt-based catalysts."
Fencing in the atoms
The team immobilized cobalt-based molecules in the micropores of zeolitic imidazolate frameworks, which served as protective fences to decrease the cobalt atoms' mobility and prevent them from clustering together. They then used high-temperature pyrolysis to convert the atoms to catalytically active sites within the framework.
Within this structure, they discovered that the density of the active sites significantly increased, in turn increasing the reaction activity. This, in fact, achieved the highest activity in fuel cells reported for non-iron, platinum group metal-free catalysts to date.
The team also found the cobalt-based catalyst to be much more durable than the iron-based catalyst synthesized using the same approach. They discovered, for the first time, significant differences in demetallation, where metal ions are leached out of the catalyst and that catalyst then loses activity. They also found that oxygen radicals from hydrogen peroxide, a byproduct of oxygen reduction in fuel cells, attack the catalysts and cause performance loss.
High activity, greater durability
"In the end, we were able to not only improve the activity of the cobalt-based catalyst, but we significantly improved the durability," said Shao. "Our further investigation led us to discover the mechanisms that typically degrade these types of catalysts."
More information: Xiaohong Xie et al. Performance enhancement and degradation mechanism identification of a single-atom Co–N–C catalyst for proton exchange membrane fuel cells, Nature Catalysis (2020). DOI: 10.1038/s41929-020-00546-1
Journal information: Nature Catalysis
Provided by Pacific Northwest National Laboratory