This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
A counterintuitive way to make stronger alloys
Humans have been mixing metals to create more useful materials for thousands of years. The Bronze Age, which started around 3300 BC, was characterized by the use of bronze, an alloy of copper and tin which is stronger than either metal alone.
Now, researchers at NTNU have discovered a counterintuitive way to make a much more recent invention—nanograined alloys, featuring nano-sized grains of the alloying element—even stronger.
Aluminum is a metal that is widely used to make components in the aerospace, transport, and construction industries, in part because it is lightweight yet durable. Alloys of aluminum retain these qualities but are stronger than aluminum alone.
"If it was a pure aluminum, of course, it's not strong enough," says Yanjun Li, Professor of Physical Metallurgy in the Department of Materials Science and Engineering at NTNU.
Large particles decrease strength
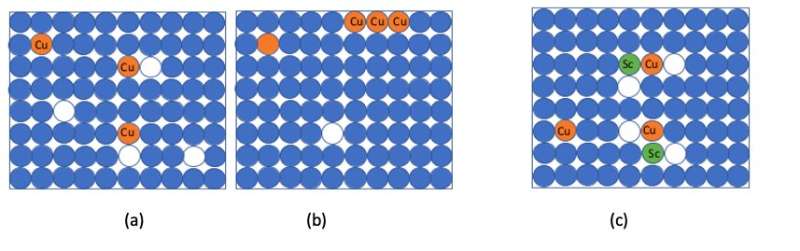
But in recent years, researchers attempting to make nanograined alloys of aluminum containing copper have run into a problem: the copper atoms have a tendency to clump together, forming coarse particles with aluminum inside the material, especially at temperatures higher than 100°C.
When the copper is no longer evenly distributed throughout the material, the alloy becomes weaker.
"They accumulate together, forming large particles," says Li. "These particles, when they are large, can actually decrease the strength."
Adding vacancies for stability
Copper atoms can move through material if there is a so-called vacancy—a space not occupied by atoms—that they can move into.
So researchers have been trying to minimize the number of vacancies to reduce the ability of atoms to move.
"If there are no vacancies, of course no atoms could move," says Li.
But now Li and his colleagues have found a way to increase the number of vacancies and at the same time increase the strength of the resulting alloy.
In work published in the journal Nature Communications, the researchers added scandium atoms as well as copper ones to aluminum, while also increasing the number of vacancies.
The scandium and copper atoms, together with the vacancies, formed structures that could not easily move through the material.
"Together they are very stable," says Li. "It becomes more difficult for any of them to move."
Thanks to the new scandium-copper structures, large aluminum-copper particles that would have previously formed were completely suppressed, even when the alloy was heated to 200°C for 24 hours.
This stability means the copper atoms stay evenly distributed throughout the material, and the alloy retains its added strength.
Atom probe tomography key
The team saw the copper-scandium-vacancy clusters using Atom Probe Tomography (APT), a technique which makes it possible to see what's happening at the atomic level inside a material.
Ph.D. student Hanne Søreide prepared very thin needles—only 50 nm in diameter—of the alloy using NTNU Nanolab's Focussed Ion Beam. She then used the atom probe to evaporate atoms, one by one, from the top of the needle, while a detector captured information about them.
"Different atoms can fly faster or slower," says Li.
Using this information, the researchers reconstructed a picture of where each atom originally was in the material. They saw that atoms of the two different alloying elements were joining together inside the aluminum.
"They are bonding together, we can detect this with copper and scandium," says Li. "They are really closely connecting together." The researchers confirmed their findings using transition electron microscopy.
50% stronger
Li says the team have already used these findings to make an aluminum alloy that is 50% stronger than alloys containing the same amount of copper that are currently available commercially.
"This is not an easy task for aluminum," he says.
While the materials Li and colleagues are making in the lab are still far from application in industry, alloys that retain their strength at high temperatures are useful in engines and for other applications where the components get hot.
NTNU's atom probe lab is the first facility of its kind in Norway. Li and his colleagues hope that using APT will help them find other new materials with desirable properties. "By really understanding the material in the atomic scale, it can help us to design new alloys, new materials with even higher strength," says Li. "Without this kind of instrument, it's almost impossible to understand these materials."
More information: Shenghua Wu et al, Freezing solute atoms in nanograined aluminum alloys via high-density vacancies, Nature Communications (2022). DOI: 10.1038/s41467-022-31222-6
Journal information: Nature Communications
Provided by Norwegian University of Science and Technology




















