This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Researchers propose copper-catalyzed rearrangement of cyclic ethynylethylene carbonates
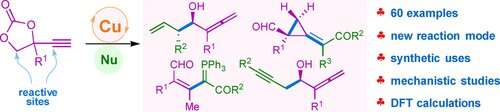
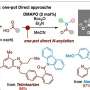
Copper-catalyzed reaction of cyclic ethynylethylene carbonates (EECs) has become one of the hottest research fields. Most of the reaction modes involve the nucleophilic substitution process. However, the development of new reactivities or reaction modes based on copper-catalyzed EECs remains a challenge.
In a study published in Angewandte Chemie International Edition, the research group led by Prof. Fang Xinqiang from Fujian Institute of Research on the Structure of Matter (FJIRSM) of the Chinese Academy of Sciences reported an unprecedented copper-catalyzed rearrangement of cyclic EECs.
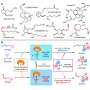
EECs release allenic aldehyde intermediates in situ, which react with diverse nucleophiles through the addition process rather than the substitution pathway. Base-mediated deprotonation is the key step of the reaction.
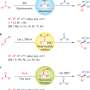
Under optimal conditions, the researchers used allylic boronates as nucleophilic reagents to obtain a series of allenols in moderate to excellent yields. Using enantioenriched boronates, they created enantioenriched allenols bearing various aromatic substituents. Propargylic boronates were also suitable nucleophiles for the synthesis of the propargylic allenols.
The researchers synthesized the highly functionalized tetra-substituted vinyl ylides in good yields with excellent stereoselectivity using phosphorus ylides as the nucleophilic reagents. The aldehyde unit of the allenic aldehyde intermediate remains intact during the conversion.
In addition, the researchers found that sulfur ylides were suitable for this reaction, with methylenecyclopropane products and the corresponding derivative of diol products generated in moderate yields. Preliminary analysis revealed that the methylenecyclopropane products were generated through the unprecedented process of C-C single bond and C=C double bond formation. This reaction provided a simple and feasible method to prepare methylenecyclopropanes with dense functional groups.
The mechanistic studies and density functional theory (DFT) calculations showed that the key step of the allenic aldehyde intermediate formation is the base-mediated deprotonation process rather than the hydrogen transfer process through the pinacol-type rearrangement.
This study represents a new reaction mode for the currently widely studied cyclic propargylic carbonates and opens a new window for broadening the utility of this type of substrate.
More information: Chao Xu et al, Copper‐Catalysed Rearrangement of Cyclic Ethynylethylene Carbonates: Synthetic Applications and Mechanistic Studies, Angewandte Chemie International Edition (2023). DOI: 10.1002/anie.202219064
Journal information: Angewandte Chemie International Edition
Provided by Chinese Academy of Sciences