Structure of a bacteriophytochrome in two states revealed
Scientist have revealed both dark adapted and light-activated structures of a red photosensory protein, phytochrome.
According to the results of the study, almost non-existent structural changes in the regulatory domains can cause large changes elsewhere. The study is published in Nature Communications.
'Nobody has succeeded in this before'
Plants and bacteria adapt to light environment by using various photoreceptor proteins. Phytochromes are a group of photoreceptors that respond to red and far-red light. The function of phytochromes have been studied extensively. Still, their full and biologically relevant structures have remained elusive.
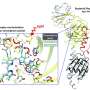
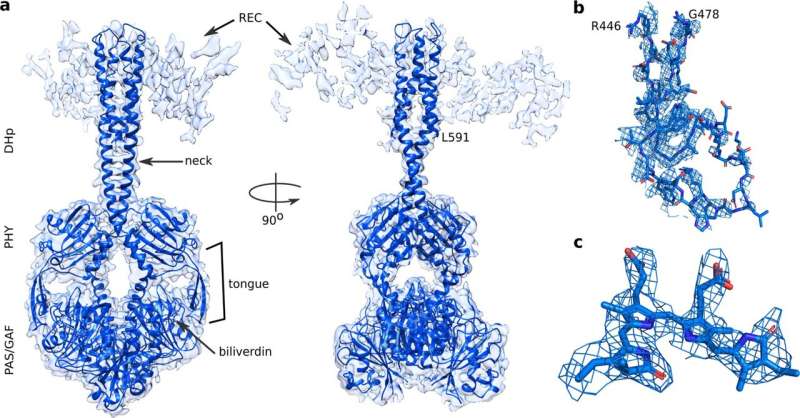
Now, full-length structures of a model bacterial phytochrome, DrBphP, from Deinococcus radiodurans have been revealed in two activity states. "Although many groups have tried, nobody has succeeded [in solving] a crystal structure of a full-length phytochrome," explains Heikki Takala from the University of Jyväskylä. "We therefore decided to apply cryo-electron microscopy to this model phytochrome."
Light-triggered structural changes revealed by cryogenic electron microscopy
An international team, including the groups of Dr. Heikki Takala and Prof. Janne Ihalainen from the University of Jyväskylä, have now successfully uncovered the structure of DrBphP.
By using single particle cryo-electron microscopy (cryo-EM), they found out that the structure of the full-length phytochrome is a symmetrical and relatively well-defined dimer in the dark-adapted state. However, when illuminated with red light, its output histidine kinase module becomes asymmetrical and less defined.
Unlike predicted in previous studies, the light-induced structural changes in the photosensory module were small but amplified only at the output module. "These results show that almost non-existent structural changes in the regulatory domains can cause large changes elsewhere, giving valuable information about signal propagation and allostery in sensory proteins," concludes Prof. Janne Ihalainen, one of the senior scientists on the team.
More information: Weixiao Yuan Wahlgren et al, Structural mechanism of signal transduction in a phytochrome histidine kinase, Nature Communications (2022). DOI: 10.1038/s41467-022-34893-3
Journal information: Nature Communications
Provided by University of Jyväskylä