Researchers realize asymmetric electrochemical radical functionalization of alkenes and allylation
In recent years, electrocatalysis has swiftly established its dominance in organic synthesis owing to its high efficiency and low contamination. However, without developed methods for controlling regio- and stereochemistry, the selectivity control proved difficult in this field.
Recently, the research team led by Prof. Guo Chang from the University of Science and Technology of China (USTC) succeeded in realizing the asymmetric electrochemical radical functionalization of alkenes and allylation. The relevant achievements were reported in Science Advances and Angewandte Chemie International Edition.
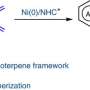
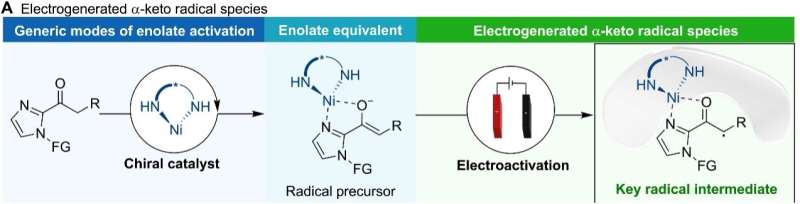
The team first activated the nucleophile with stereoselective Lewis acid-Nickel complex to form a key radical intermediate through oxidation on the anode.
The intermediate was then added to various alkenes to form new radicals that ultimately turned into alkene difunctionalization (Path1) or alkenylation (Path2) products through further oxidation and addition (or elimination). The products analysis showed high asymmetric selectivity thanks to the single electron-transfer (SET) process and the Nickel catalyst.
The researchers carried out extensive experiments on the function and necessity of each reaction component in order to better understand the mechanism of the asymmetric electrolytic reaction. They discovered that the electrode potentials of the nickel-mediated alkene oxidation are significantly lower than those of the direct oxidation.
Moreover, using similar methods, the team developed an electrocatalytic asymmetric allylation in which the intermediate was an electron-deficient α-ketone radical.
"We believe that in the near future, the realization of enantioselective electrochemical transformations will improve the scope of electrosynthesis and pave the way for exploring new chemical spaces and developing solutions to challenging synthetic problems," say the researchers.
More information: Kang Liang et al, Nickel-catalyzed switchable asymmetric electrochemical functionalization of alkenes, Science Advances (2022). DOI: 10.1126/sciadv.add7134
Qinglin Zhang et al, Enantioselective Nickel‐Catalyzed Electrochemical Radical Allylation, Angewandte Chemie International Edition (2022). DOI: 10.1002/anie.202210632
Journal information: Angewandte Chemie International Edition , Science Advances
Provided by University of Science and Technology of China