Visible light activation enables transformation of bench-stable sulfones to valuable glycosides
National University of Singapore chemists have developed a new strategy to generate therapeutically relevant C-glycosides and S-glycosides through a catalyst- and transition-metal-free approach under visible light illumination at ambient temperature. Their research appears in Nature Synthesis.
Glycosides play an indispensable role in diverse physiological functions and are found in a wide variety of natural products and synthetic compounds. C-glycosides are an important class of glycosides, which consist of a sugar-containing unit joined to an organic moiety or another sugar-containing compound, through a carbon-carbon (C-C) bond. They possess a myriad of biological activities and are structurally diverse. One convenient way to construct such products involves the direct union of a glycosyl precursor (donor) with a carbon-based reagent.
However, the scope of C-glycosides that can be accessed using currently reported methods is severely limited. This owes to the lack of practical glycosyl donors available to facilitate mild C-C coupling. A general class of bench-stable glycosyl precursors that can be readily synthesized and isolable on large scale, and yet sufficiently reactive to undergo expeditious and stereoselective cross-coupling at ambient conditions is highly desirable but elusive.
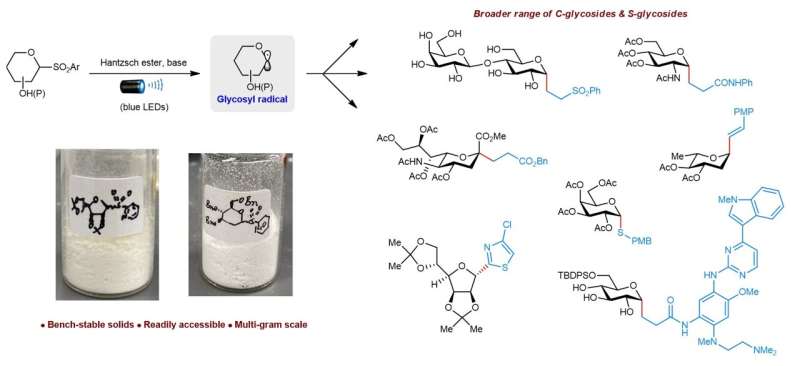
A research team led by Assistant Professor Koh Ming Joo, from the Department of Chemistry, National University of Singapore has devised robust procedures to synthesize solid heteroaryl glycosyl sulfones on multi-gram scale. The team discovered that these bench-stable sulfones are also redox-active. By using visible (blue) light illumination and a Hantzsch ester-base complex, the researchers are able to activate the sulfones to produce chemically reactive glycosyl radicals.
These radicals react readily with various electrophiles. With this method, they are able to obtain a wider range of valuable C-alkyl, C-alkenyl, C-alkynyl, C-heteroaryl and S-linked glycosides in an efficient and highly selective manner. The researchers also used ultraviolet/visible absorption spectroscopic and radical clock studies to gain insights into the mechanism of these transformations.
Prof Koh said, "This catalyst- and transition metal-free method effectively overcomes previous limitations in scope, scalability and glycosyl donor instability."
"We expect this general class of glycosyl precursors and their newly discovered reactivity under visible light illumination to find extensive utility in various carbohydrate synthetic applications, enhancing efforts towards the discovery of new sugar-based therapeutics and our understanding of biological processes," added Prof. Koh.
The research team plans to work with companies to utilize these findings for the synthesis of sugar-derived compounds.
More information: Quanquan Wang et al, Visible light activation enables desulfonylative cross-coupling of glycosyl sulfones, Nature Synthesis (2022). DOI: 10.1038/s44160-022-00162-w
Journal information: Nature Synthesis
Provided by National University of Singapore