Researchers discover method for preventing limescale
From cloudy glasses to deposits in dishwashers—limescale is a ubiquitous problem. An international research collaboration led by two researchers at FAU has now investigated which substances could be added to dishwasher detergent to prevent the build-up of limescale. Knowledge about the mechanisms involved can be used to develop more sustainable ingredients. The results have been published in the journal Angewandte Chemie.
Limescale crystals that occur before limescale, such as the milky film that remains on glasses, form when water dries and leaves a solid residue behind. Phosphates are added to dishwasher powder to tackle this problem and prevent these deposits in dishwashers. However, phosphates are also fertilizers and are transported sooner or later to rivers and oceans via the waste water. There, the phosphates make the water rich in nutrients. This allows certain types of algae to reproduce too quickly, leading to an unwanted algae plague, which can in turn lead to other organisms and plants dying en masse.
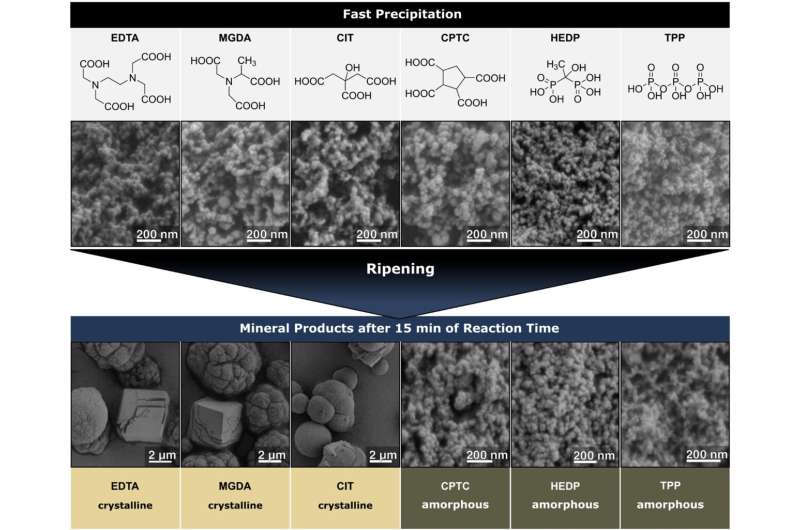
In order to abstain from using phosphates in future, the researchers from FAU led by Priv. Doz. Dr. Stephan E. Wolf, Chair of Glass and Ceramics, and Prof. Dr. Dork Zahn, Professorship of Theoretical Chemistry, have investigated how phosphates and other common ingredients prevent residues. They have discovered various mechanisms. They were able to demonstrate how exactly the complex mechanisms work and how they perfectly complement each other.
Their work led to a suggestion for a substance that functions very similarly to phosphate, but which seems to be more environmentally friendly. The substance interferes with the deposits, making them more disorganized and preventing organized crystals forming stable packages with calcium and carbonate ions. This either prevents deposits altogether or makes them easier to dissolve due to their disorganized structure.
The team was not only able to determine which mechanisms prevent limescale deposits, but also gain new insights into how lime is formed. "We discovered that the process behind forming lime is much more complex than we imagined," explains Prof. Dr. Dirk Zahn.
The new insights also help us understand the effect organisms have on lime production in nature. Crustaceans, for example, have to be able to control lime production to form their shells and ensure their survival. "Especially in view of climate change, we must understand what is particularly detrimental to lime-secreting organisms such as corals, shells and marine gastropods if we are to protect them sufficiently," says Dr. Stephan E. Wolf.
More information: Patrick Duchstein et al, Small‐Molecular‐Weight Additives Modulate Calcification by Interacting with Prenucleation Clusters on the Molecular Level**, Angewandte Chemie International Edition (2022). DOI: 10.1002/anie.202208475
Journal information: Angewandte Chemie International Edition , Angewandte Chemie
Provided by Friedrich–Alexander University Erlangen–Nurnberg