Study reveals novel mechanism behind epilepsy and drug modulation
Epilepsy is a neurological disorder that arises from abnormal electrical activity in the brain leading to seizures. These seizure events can have a variety of causes, including genetic variants in a family of proteins that regulate potassium ions in the brain. Researchers at Washington University in St. Louis have led an international team to take a close look at the mechanisms behind the function and dysfunction of these proteins, as well as their interactions with an antiepileptic drug, to develop a potential new strategy to treat epilepsy.
Jianmin Cui, professor of biomedical engineering in the McKelvey School of Engineering, and Nien-Du Yang, a doctoral student in biomedical engineering who conducts research in Cui's lab, teamed up with Harley Kurata, associate professor of pharmacology at the University of Alberta, and investigated the working mechanism of two potassium ion channels, KCNQ2 and KCNQ3. Their findings uncover a conserved mechanism for KCNQ channel activation that is a target of both epilepsy-linked mutations and a small molecule compound.
The work was published July 20 in Science Advances.
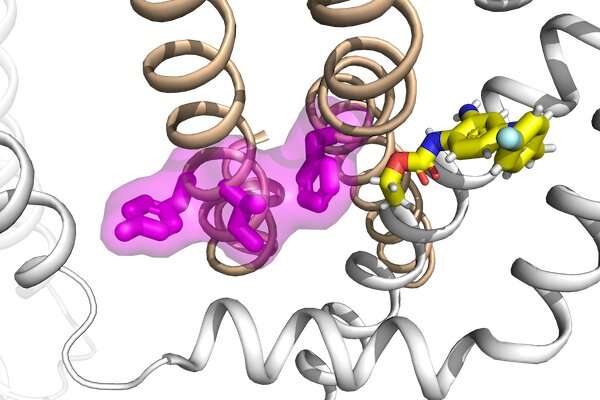
The KCNQ potassium channel family has multiple functions, from regulating heartbeat (by KCNQ1) to controlling excitability of neurons (by KCNQ2-5). These channels are voltage-activated so that they sense voltage changes across the cell membrane and open and close in response. The communication between voltage sensing and channel pore opening is known as electro-mechanical coupling, a process involving conformational changes of the protein during voltage-dependent activation.
Cui's team has previously shown that KCNQ1, the cardiac KCNQ isoform, features a two-stage process in electro-mechanical coupling that leads to two distinct channel open states, the intermediate-open and activated-open. Regulation of the two open states underlies KCNQ1's tissue-specific modulations, disease pathogenesis and pharmacology. KCNQ2 and KCNQ3 are highly expressed in the central nervous system and are the principal contributors to the M-current, a critical potassium current that modulates neuronal excitability. Therefore, impaired M-current function by congenital mutations in KCNQ2 and KCNQ3 is commonly associated with early-onset and pediatric epilepsy.
"Although KCNQ channels are highly similar in their sequences and structures, whether the neuronal KCNQ isoforms also share the same electro-mechanical coupling mechanism or two open states is unclear," said Yang, the paper's first author. "This work reveals key similarities and differences between these channels that may have important implications for their function in cardiomyocytes or neurons."
The team used a variety of methods to study the electro-mechanical coupling mechanism in these potassium channels, including creating specific genetic mutations in the channels, electrophysiology and fluorescence optical measurements.
"Elucidating the molecular mechanism for electro-mechanical coupling is an important step toward understanding the voltage-dependent gating of potassium channels," Cui said. "We provided functional evidence that the neuronal KCNQ2 and KCNQ3 channels are different from KCNQ1 in which they feature a single activated-open state but with a conserved electro-mechanical coupling mechanism specific for the activated-open state."
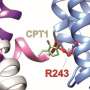
These channels are prime targets for treatments for epilepsy, the researchers found. The team also identified a set of mutations in KCNQ2 and KCNQ3 associated with early infantile epileptic encephalopathy, a severe form of childhood epilepsy, that specifically disrupts the electro-mechanical coupling of the channels. The researchers took advantage of an antiepileptic prototype drug retigabine given its mechanism of action on neuronal KCNQ channels and demonstrated that the electro-mechanical coupling can be directly enhanced to rescue the function of these diseased mutants. Their studies suggest that the electro-mechanical coupling mechanism in KCNQ channels can be an effective target, presenting a novel pharmacological strategy for developing more effective therapies for epilepsy treatment.
More information: Nien-Du Yang et al, Electro-mechanical coupling of KCNQ channels is a target of epilepsy-associated mutations and retigabine, Science Advances (2022). DOI: 10.1126/sciadv.abo3625
Journal information: Science Advances