Key to growing natural tissue for female reproductive system reconstruction unlocked

Researchers have developed a ground-breaking technology to culture natural tissue from female fetal fallopian tubes and uteri, potentially paving the way to help women born with reproductive abnormalities to attain normal function through the growth of their own cells.
One of the fascinating events in human development is the transformation of Mullerian ducts, a set of simple and uniform tubes, into spatially restricted and highly complex reproductive organs. This process occurs in a relatively short window of time during fetal development, however any irregularities result in females born with missing or defective organs.
With up to 80,000 female reproductive repair surgeries performed every year in the United States alone, Professor Pradeep Tanwar and his team from HMRI and the University of Newcastle, committed to unraveling the mysteries of human reproductive tract development.
Their findings are published in the Proceedings of the National Academy of Sciences.
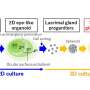
To create the natural tissue the researchers successfully grew organoids—tiny, self-organized three-dimensional tissue cultures that are derived from stem cells, in this instance, from human fetal fallopian tubes and uteri.
In growing the organoids, the team replicated what occurs in nature by studying the key stages of human fetal development, where the female reproductive tract is progressively derived from the growth and fusion of the two Mullerian ducts.
They discovered grafting these fetal organoids onto scaffolds, prepared from native adult tissue taken from a patient, could regenerate normal tissue, ex-vivo. The finding could lead to reconstructive surgeries being performed on patients with reproductive abnormalities through growing their own cells.
Professor Tanwar said there was a huge variety of reproductive abnormalities and a need to improve how these are rectified to benefit quality of life.
"Our study has laid the foundation for growing patients' own cells and using them for regenerative surgeries to reduce future complications and improve the quality of life for these patients," Professor Tanwar said.
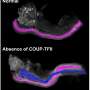
As part of this process, the researchers discovered a group of proteins called WNT, determining they are essential for normal reproductive tract development. Alterations in these proteins are what causes reproductive system abnormalities in the first place. The team showed that without WNT proteins, the Mullerian ducts that form during the first six weeks of gestation do not develop normally.
The abnormalities in WNT proteins are found in women with a condition called Mayer-Rokitansky-Küster-Hauser syndrome (MRKH)—a genetic disorder that presents in one in 4500 women. MRKH syndrome is just one of the conditions that can lead to reproductive abnormalities and the subsequent complications and/or need for surgical repair later in life.
More information: Varshini D. Venkata et al, Development and characterization of human fetal female reproductive tract organoids to understand Müllerian duct anomalies, Proceedings of the National Academy of Sciences (2022). DOI: 10.1073/pnas.2118054119
Journal information: Proceedings of the National Academy of Sciences
Provided by Newcastle University