Biomaterials for kidney organoid–based regenerative therapy
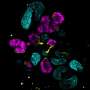
![Renal microstructures found in kidney organoids. Immunofluorescent microscopy images of all nephron segments found in kidney organoids generated using Takasato protocol. Figure adapted from reference [3]. Credit: https://research.tue.nl/en/publications/biomaterials-for-kidney-organoid-based-regenerative-therapy Biomaterials for kidney organoid–based regenerative therapy](https://scx1.b-cdn.net/csz/news/800a/2022/biomaterials-for-kidne.jpg)
With the discovery of synthetic stem cells, better known as induced pluripotent stem cells, the field or regenerative medicine has been revolutionized. These synthetic stem cells are created by reprogramming adult cells from patients, such as human skin cells, blood cells, or urine cells, toward a pluripotent state—a cell that can become any type of cell in the human body. When forced to be one type of cells, these cells can become tiny versions of organs known as organoids. For his Ph.D. research, Johnick van Sprang developed an artificial nano-environment using next-generation materials based on supramolecular interactions, to control and refine organoid maturation through biomechanical cues.
Organoids
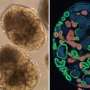
When induced pluripotent stem cells (iPSCs) are grown in a three-dimensional culture using specific biological cues, they develop into miniature organs known as organoids. These iPSC-derived organoids demonstrate high resemblance to adult organs in terms of microstructures and function.
Organoids that function like renal tissue (i.e., kidney organoids) are proposed as an alternative to donor kidney transplantation in the form of a personalized and regenerative therapy. However, the process of training iPSCs to be kidney organoids is still imperfect and requires further refinement. As such, additional strategies are needed that offer novel cues to iPSCs to control their specialization. One way to achieve this is through the use of biomaterials, which can provide biomechanical cues to cells. This is lacking in cell differentiation strategies that only rely on soluble, biochemical agents.
Increasing evidence suggests that changes in the mechanical environment have drastic effects on the development of kidney organoids, which allow improvement of functional structures and even decrease the onset of off-target cell types. Changing the mechanical environment is possible by switching from an air-liquid interface culture to embedding or encapsulating organoids in a hydrogel material.
Artificial nano-environment
As part of his Ph.D. research, Johnick van Sprang aimed to develop an artificial nano-environment, using next-generation materials based on supramolecular interactions, to control organoid growth through the use of biomechanical cues.
Supramolecular materials are held together by non-covalent bonds, which are interactions between molecules with a relatively low energy (e.g., hydrogen bonds, hydrophobic interactions, and electrostatic interactions). The low energy of the bonds makes them more susceptible to breaking and reforming compared to chemical bonds. In turn, this results in an adaptable material with dynamic properties.
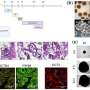
In his work, van Sprang engineered different types of molecular building blocks containing the supramolecular moiety ureido-pyrimidinone (UPy) into the artificial nano-environments that were used to encapsulate kidney organoids. These UPy molecules recognize each other and self-assemble into fibrous superstructures, which resemble the fibrillar components of natural tissue.
Tuning
By combining different UPy molecules, van Sprang was able to engineer the degree of biochemical complexity, mechanical properties of the artificial nano-environment, and the speed at which the molecules switched from solution to a gel. By encoding a delayed transition from a solution to a gel state, it was possible for the artificial nano-environment to enter the kidney organoids as fibrillar superstructures while still in the solution state.
This enabled the artificial nano-environment to elicit a biological response beyond the organoid-gel interface, and also deliver biomechanical cues to cells within the organoid. The result was a three-fold increase in glomeruli, which are the functional structures of kidneys that filter blood.
This demonstrated that it is possible to tune the specialization of cells within kidney organoids using biomaterials that provide mechanical cues in a complementary fashion to soluble, biochemical agents. This discovery provides novel opportunities to gain more control over specialization and maturation of kidney organoids, which may bring kidney organoids one step closer to the clinic.
More information: Biomaterials for kidney organoid-based regenerative therapy. research.tue.nl/en/publication … regenerative-therapy
Provided by Eindhoven University of Technology