Study probes how DNA folding might affect gene activity
Russian researchers from Skoltech, the Institute of Molecular Genetics of NRC Kurchatov Institute, Lomonosov Moscow State University, and elsewhere have clarified the mechanism behind the activation of genes in drosophila fly sex cells transitioning between two early stages in spermatozoid development. A similar mechanism makes the cells in our body—in the muscles, nerves, liver, etc.—different from each other and perhaps from sick cells, too. The team's findings were published in Nucleic Acids Research, about how the 3D structure assumed by DNA regulates which genes are active. These findings are a step toward elucidating the mechanisms of diseases and their causes with regard to DNA packaging.
Unless you have an unusual genetic condition or an organ transplant, all the cells in your body except the reproductive ones carry the same DNA. What makes brain cells look and behave differently from, say, heart cells is the particular set of genes in the DNA that are activated based on the type of tissue and the stage of development.
The mechanisms behind this so-called gene regulation are still unknown, but scientists suspect they have a lot to do with how DNA is packaged inside a cell nucleus. "DNA is a 2-meter-long molecule crammed inside a cell nucleus, which is about one-hundredth of a millimeter across. Depending on the particular 3D configuration assumed by the DNA, regions carrying different genes are either available for activation by proteins or buried where no protein will reach them, rendering them inactive," explains Skoltech Assistant Professor Ekaterina Khrameva.
If DNA packaging is how cells regulate what genes are active, then abnormal packaging can result in misregulation and cause disease. She says, "So far, there are few diseases definitively linked to incorrect DNA packaging, but there are probably many more. It's just that the technique used to map DNA conformation—the spatial configuration of the molecule inside the nucleus—has only been around since 2009."
Anna Kononkova, a junior research scientist, says, "The problem is it is not enough to detect DNA conformation in a healthy cell and contrast it with that in a sick cell. To understand the mechanism behind such a disease and figure out ways of treating it, scientists have to know precisely what effect incorrect packaging has on gene functioning in each particular disorder."
"So far, we are trying to understand the general rules that govern how DNA packaging affects gene regulation. The model we're using is the drosophila fly, and specifically, its immature sex cell called spermatogonium as it develops into a spermatocyte, a pre-spermatozoid of sorts," she elaborates.
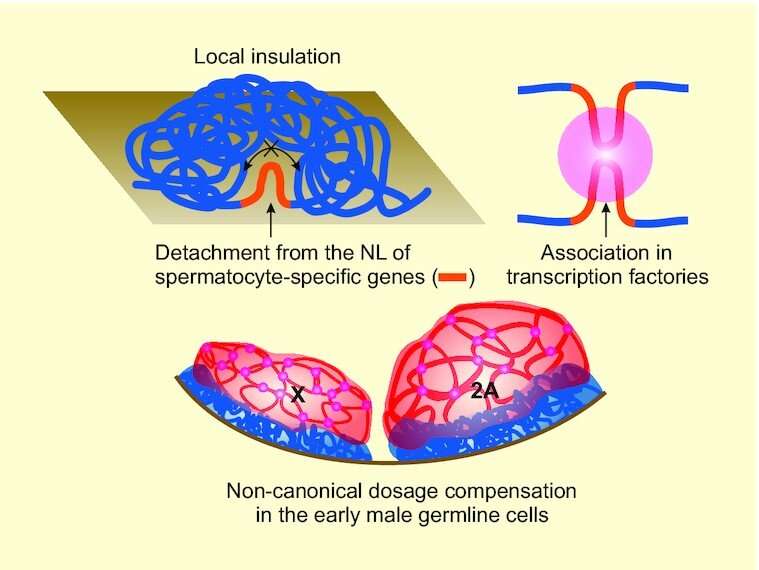
Assistant Professor Ekaterina Khrameva says, "Spermatogonia and spermatocytes are thus two different stages in the development of the same cell. In transitioning between these stages, as many as 1,000 genes are activated. This means that whatever is responsible for their regulation must kick into high gear at that time. So if changes in DNA packaging are at play, they should be detectable. Indeed, we observed that the DNA regions housing the activated genes tended to loosen up their structure. This agrees well with the intuition that they should be 'exposed' to facilitate the approach of activating proteins."
The researchers also noted that the shape of the DNA molecule changed in such a way that the activated genes tended to come together in a number of specific locations. This supports the hypothesis that RNA synthesis—that is, copying information from active genes for making proteins—does not happen in arbitrary places throughout the cell nucleus. Rather, it occurs at specific spots called transcription factories.
To achieve these results, the team carried out computationally intensive experiments using a DNA conformation analysis technique known as Hi-C. It allowed the researchers to quantify the likelihood of finding every small fragment of DNA next to every other one. So rather than produce a pretty 3D picture of how the DNA molecule is folded in the nucleus, the method yields a bunch of numbers, but to a qualified specialist those are more or less equivalent.
By documenting the changes in DNA packaging that take place in conjunction with a massive activation of genes, the researchers have made one more step toward uncovering gene regulation mechanisms and understanding the associated diseases. One of the questions for further research to address would be to see if drosophila fly genes come into contact with each other more often than random fragments of DNA.
More information: Artem A Ilyin et al, Comparison of genome architecture at two stages of male germline cell differentiation in Drosophila, Nucleic Acids Research (2022). DOI: 10.1093/nar/gkac109
Journal information: Nucleic Acids Research
Provided by Skolkovo Institute of Science and Technology