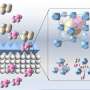
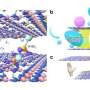
CO2 separation by special membranes

Worldwide, CO2-emissions must be reduced drastically and one way is separation of CO2 from industrial waste streams. These membranes do not function properly at high pressure conditions however. Chemical engineer Menno Houben found the cause and optimized special membranes that allow separating CO2 at high pressure. Friday November 19th he will defend his Ph.D. thesis at the department of Chemical Engineering and Chemistry.
To prevent the worst impacts of climate change, the amount of atmospherical CO2 should be reduced. CO2 could be separated from natural gas and biogas, but also from industrial waste streams, to minimize atmospheric pollution. Diverse polymeric membranes are already developed for this kind of separation. However, these membranes do not function properly at high pressure conditions and therefore Ph.D.-student Menno Houben investigated how a more efficient capture of CO2 could be realized.
Membranes without pores
If you want to separate CO2 using a membrane, you need pressure difference, Houben explains. "At a higher pressure, subsequently more gas will flow through the membrane. You might think of a kind of coffee filter containing microscopic pores, but the membranes we use for gas separation are quite different. They do not contain any pores and are often called dense membranes."
"These membranes can have a very high separation efficiency, but when the gas pressure goes up, problems arise and suddenly they are a lot less efficient. Tricky, because you need high pressure to keep productivity high. And natural gas, for example, is also under a high pressure in the ground."
Swelling membrane
According to Houben, the problems arise from plasticization, the swelling of the membrane due to the absorption of a lot of CO2. At higher pressures in particular, the membrane swells quickly and therefore functions much less well.
In addition, CO2 under high pressure is in the supercritical phase. In this manifestation, the distinction between gas and liquid phase has disappeared and it has unique properties. Supercritical CO2 only occurs at relatively high temperature and pressure, precisely conditions in which separation membranes quickly plasticize.
Heat treatment
Houben investigated the plasticization process at molecular level and found that especially at supercritical conditions, the membrane's performance is mainly determined by the balance between the liquid properties of CO2 and plasticization.
"We also saw that stable membranes are a lot more resistant to plasticization and so the membrane remains well separated for longer. We obtained these stable membranes in three different ways: by mixing polymers, a heat treatment and by chemically cross-linking the membranes. All methods proved effective, but the membranes that had undergone heat treatment gave the most favorable properties."
"This allowed us to make a stable membrane that works well at high pressures and does not plasticize as quickly. Now admittedly only on a lab scale, but hopefully these insights can start to be used in the development of new stable membranes for high-pressure applications."
Menno Houben will defend his thesis "Critical aspects of high-pressure CO2-induced plasticization in polyimidemembranes" on Friday, November 19.
Provided by Eindhoven University of Technology