Researchers model circadian clock neurons in a day-active animal for the first time

It's no secret that jet lag and night-shift work can wreak havoc on the way our body's internal clock syncs up our daily wake-sleep cycle, known as circadian rhythm, but now researchers say they are a step closer to understanding how the brain creates behavioral rhythms optimized for diurnal, rather than nocturnal, life.
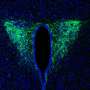
In a new study published Nov. 30 in the journal eLife, researchers have reported the first-ever recording and modeling of the electrical activity of circadian clock neurons in a diurnal species—the four-striped grass mouse, Rhabdomys pumilio.
Until now, brain recording studies of nocturnal species have primarily been used to form an understanding of the mammalian master circadian clock—located in the brain's hypothalamic suprachiasmatic nucleus (SCN), where nearly 20,000 neurons synchronize with the light-dark cycle via electrical signals to orchestrate circadian rhythms in our physiology and behavior.
Researchers say the study is an advance toward more precisely exploring the connection between circadian rhythms and human health, including the relationship between daytime light exposure and circadian clock-related sleep disorders.
"Almost everything we know about the brain's circadian clock comes from studies on night-active rodents such as rats and mice, which complicates translating this knowledge to human circadian rhythms," said Casey Diekman, co-corresponding author of the study and mathematical biologist at New Jersey Institute of Technology. "This work is the first to describe the intricate electrical landscape of the SCN in a diurnal mammal, and it's highlighted notable differences from nocturnal animals that may be important in adapting clock neuron function to the specific biological demands of a day-active species."
"We found that the overall day/night pattern of SCN neuron activity in the diurnal rodent R. pumilio is similar to the pattern previously observed in night-active species," said Beatriz Bano-Otalora, co-first author of the paper and a biologist working with the labs of Robert Lucas and Timothy Brown at the University of Manchester. "We've also found unique features in how R. pumilio's SCN neurons behave that have never been observed before in nocturnal species."
The team found that like nocturnal rodents, R. pumilio's SCN neurons spontaneously fired at a higher rate during daytime hours than at night. This day/night rhythm in firing rate is the main signal the SCN sends to the rest of the brain to communicate the time of day.
"However, when we injected currents to inhibit these neurons, some cells exhibited a pronounced delay before resuming to fire after inhibition was released," explained Mino Belle, co-corresponding author of the paper and a biologist at the University of Exeter. "This delay-to-fire response is not present in the SCN of nocturnal rodents and may affect how R. pumilio clock neurons respond to inputs they receive from other cells."
To learn more, the team combined the voltage traces recorded from the rodent's brain with a newly developed data assimilation algorithm. They built computational models simulating the complex interaction of voltage-gated ion channels that produce action potentials. The simulations showed that increased conductivity of a particular ion channel, the transient A- potassium channel, was responsible for the delay-to-fire response.
"The enhanced conductance of this potassium channel that our models pointed out could be advantageous for a diurnal species," said the paper's co-first author Matthew Moye, a postdoctoral fellow at Merck & Co. who began developing the team's data assimilation algorithms as a Ph.D. student in NJIT's Department of Mathematical Sciences. "Wakefulness results in inhibitory behavioral feedback signals to the SCN, which in nocturnal animals helps keep SCN firing rates low at night. In diurnal animals, this nighttime inhibitory feedback is not present, so enhanced A-type conductance may be needed to silence the SCN at night and preserve the overall day/night firing pattern."
The team's research follows separate findings from Diekman and colleagues at Northwestern University recently published Nov. 15 in Proceedings of the National Academy of Sciences, which revealed the role of the gene Tango10 as a critical link between the circadian clock and the production of daily wake-up signals at the cellular level. Diekman says the same data assimilation method developed to study R. pumilio neurons was used to construct mathematical models from voltage traces of the fruit fly Drosophila melanogaster, ultimately showing how Tango10 gene mutations contribute to disruptions in daily rhythms.
"Now that we have this powerful tool for extracting information from voltage traces, we hope to continue collaborating with electrophysiology labs and apply data assimilation to recordings not just from circadian clock neurons, but also from neurons that are associated with neurodegenerative diseases such as Alzheimer's and Huntington's," Diekman said.
More information: Daily electrical activity in the master circadian clock of a diurnal mammal, eLife, DOI: 10.7554/eLife.68179 , elifesciences.org/articles/68179
Journal information: eLife , Proceedings of the National Academy of Sciences
Provided by New Jersey Institute of Technology