Scientists identify protein that stops cell cycle in response to stress
UT Southwestern researchers have identified a new mechanism by which stress causes cells to stop dividing.
Using techniques created at UT Southwestern, the team identified a protein in yeast that plays a previously unrecognized role in halting the cell cycle, the process by which one cell reproduces itself by splitting into two. After accumulating in response to stressful events, the Xbp1 protein appears to suppress the cell cycle, the study suggests.
The findings, published in the Journal of Cell Biology, appear to solve one long-standing mystery of the cell cycle. If scientists can identify a protein in mammals with a similar function, the research might eventually lead to new ways to accelerate wound healing by encouraging cell division or improve cancer treatment by doing the opposite.
"Scientists have been studying this fundamental process for nearly seven decades," said Orlando Argüello-Miranda, Ph.D., an Instructor who co-led the study with Assistant Professor Jungsik Noh, Ph.D., both of UTSW's Lyda Hill Department of Bioinformatics. "We've essentially identified a protein that can stop the cell cycle in response to stressful conditions."
Scientists have long known that the cell cycle consists of several defined stages as a cell goes from a resting state to increasing in size as it copies its genetic material—or DNA—in a process known as DNA replication, condenses its DNA, and finally divides into two "daughter" cells.
It has long been known that a stressful event such as starvation can send a cell into a protective state known as quiescence. Typically, the cell cycle halts just before DNA replication takes place, Dr. Argüello-Miranda explained. However, a minority of cells seem to become quiescent at other points in the process.
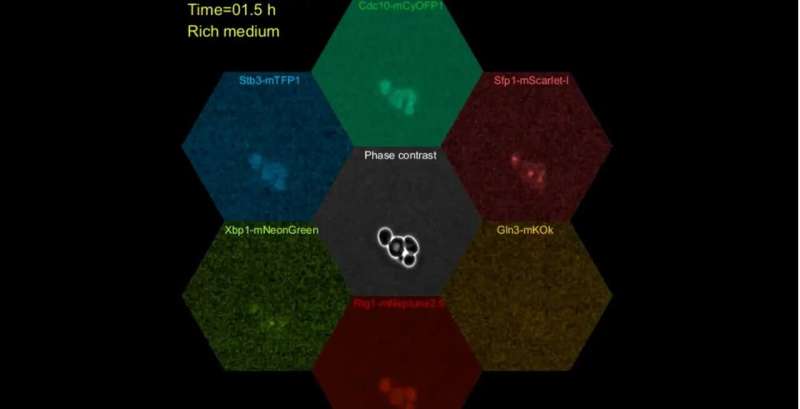
To understand why, Dr. Argüello-Miranda, Dr. Noh, and their colleagues studied yeast cells challenged by nutritional stress. The researchers were able to study individual cells in that state by using a technique called microfluidics six-color imaging developed at UT Southwestern. That technique combines a technology for cell culture called microfluidics with a six-color fluorescent-microscopy approach pioneered at UTSW in 2018. In microfluidics six-color-imaging, investigators pass cells through a tiny, fluid-filled chamber monitored with a specialized microscope and camera array. The researchers also used machine learning to track and detect up to six biochemical reactions in single cells in real time.
"In previous studies in the field, researchers cultured yeast cells in flasks and were unable to track single cells," Dr. Argüello-Miranda said. "In contrast, we have obtained movies that record how individual cells stop dividing and enter quiescence."
Although most of the starved cells entered quiescence at the expected stage, right before DNA replication, about 7% paused at another stage of the cell cycle, he said.
Using the new techniques to tag and follow specific proteins in individual cells over time, the researchers found that all the starved cells showed elevated levels of a suite of stress response proteins. However, cells that became quiescent at unexpected points in the cell cycle all had an abundance of Xbp1, which the study found is needed to stop the cell cycle after DNA replication.
Further experiments showed that this accumulation of Xbp1 caused the cell cycle to pause, even when the activity of another protein called Cdk1 that encourages cell proliferation is high. A closer look showed that Xbp1 levels weren't static—rather, they climbed higher after each stressful event an individual cell experienced or the longer a stressful event lasted.
"This accumulation was so predictable we could tell how many stressful events a cell had been exposed to by how much Xbp1 was present in the cell nucleus," Dr. Argüello-Miranda said. The findings suggest that Xbp1 has a newly discovered function in regulating the cell cycle, allowing yeast cells to "remember" exposure to stress and to protect themselves by entering quiescence, he added.
Although there is no direct homolog to Xbp1 in mammals, other proteins seem to show similar biochemical activity in mammalian cells. Those proteins will be the subject of future research, he said.
More information: Orlando Argüello-Miranda et al, Cell cycle–independent integration of stress signals by Xbp1 promotes Non-G1/G0 quiescence entry, Journal of Cell Biology (2021). DOI: 10.1083/jcb.202103171
Journal information: Journal of Cell Biology
Provided by UT Southwestern Medical Center