Prime mechanism for antimicrobial resistance in gonorrhea analyzed with eye toward new treatments
Sexually transmitted infection (STI) gonorrhea is on the rise as a major public health burden worldwide, with around 87 million new infections a year largely caused by the superbug Neisseria gonorrhoeae which experts fear will soon be untreatable
In a new paper, published in mBio, scientists at Flinders University and the Australian National University have analyzed the prime mechanism for antimicrobial resistance in this crafty organism—paving the way for further developments in treatment options.
"Antimicrobial resistance in Neisseria gonorrhoeae has reached an alarming level," says lead author Flinders University Professor of Microbiology Melissa Brown.
The World Health Organization has ranked N. gonorrhoeae as one of 12 antimicrobial resistant bacterial species that poses the greatest risk to human health, motivating medical researchers around the world to pursue alternative treatments.
"We need to find the strengths and weaknesses in these species and in this study we have focused on the manner by which drugs are pumped out of these cells which helps the superbug become more resistant and able to survive treatment by multiple drugs," Professor Brown says.
"Such treatment failures subsequently lead to increased medical costs and a decrease in human general and reproductive health."
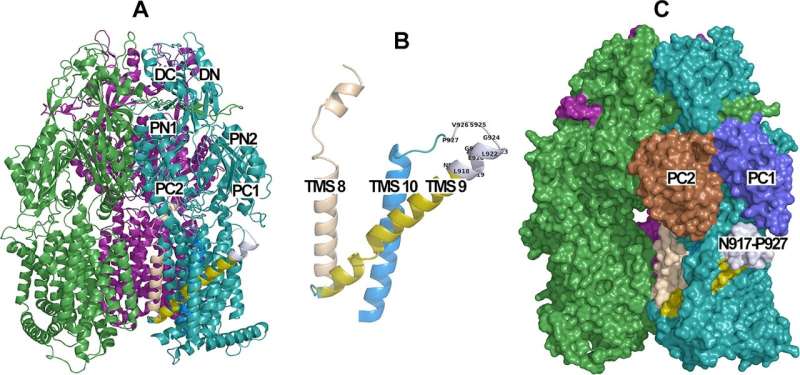
Together with ANU colleagues led by Associate Professor Megan O'Mara, the Australian research team has identified a region unique to the drug pump that plays a role in positioning the protein in the surface of the bacteria enabling it to function optimally.
"This could be a future target for antibiotic or antimicrobial development," says first author on the new paper Mohsen Chitsaz, whose Ph.D. study at Flinders University is supported by an Australian Government Research Training Program Scholarship.
More information: Mohsen Chitsaz et al, A Unique Sequence Is Essential for Efficient Multidrug Efflux Function of the MtrD Protein of Neisseria gonorrhoeae, mBio (2021). DOI: 10.1128/mBio.01675-21
Journal information: mBio
Provided by Flinders University