Understanding a nanomuscle
Cells in the human body constantly receive substances from the outside via a process called endocytosis. One important endocytosis pathway involves the formation of a protrusion within the cell membrane, pointing to the inside of the cell, triggered when a molecule needing to get in reaches the membrane. The protrusion wraps and closes around the molecule, after which it is cut off, leaving the wrapped molecule (a so-called vesicle) inside the cell. A key role in the cutting-off mechanism is played by dynamin, a protein that can locally 'constrict' cell membranes, and chop off bits. The precise constriction mechanism is not completely understood, however. Now, by combining experiments and simulations probing the dynamics of dynamin, Alexander Mikhailov from Kanazawa University and colleagues show that the workings of such a 'nanomuscle' resembles that of a ratchet motor.
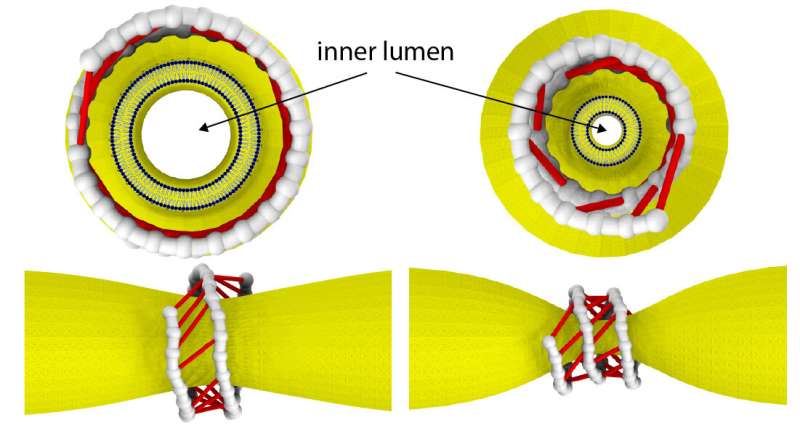
Dynamin consists of helical filaments, which coil around the neck of a vesicle forming within a membrane. In the presence of GTP (a molecule that can be thought of as a biochemical 'fuel', as it provides energy) these filaments start to slide, and in doing so constrict the vesicle neck. In order to gain detailed insights into the constriction mechanism, Mikhailov and colleagues used a technique called single molecule fluorescence resonance energy transfer (smFRET), which enables measuring distances in biomolecules. The obtained data revealed that different conformations of dynamin's filaments occur, depending on whether or not they are bound to GTP and its products. These various configurations result in the formation of differently oriented molecular 'bridges' between neighboring coil turns. Supplying GTP and hydrolyzing it induces power strokes in the cross-bridges and generates a filament torque.
The scientists then designed a computable model of a set of dynamin filaments wrapped around a membrane protrusion, capturing the structural changes and their timescales as observed in the smFRET measurements. The membrane was modeled as a deformable cylindrical tube, and the filaments as strings of beads connected to active cross-bridges powered by GTP. The simulations showed that the combined motion of cross-bridges provides a force large enough to cut a membrane tube.
The work of Mikhalov and colleagues confirms, in a quantitative way, the constriction-by-ratchet model of the dynamin nanomuscle—nature's strongest torque-generating motor. The researchers explained that their "experimental and computational study provides an example of how collective motor action in megadalton molecular assemblies can be approached and explicitly resolved."
More information: Oleg M. Ganichkin et al, Quantification and demonstration of the collective constriction-by-ratchet mechanism in the dynamin molecular motor, Proceedings of the National Academy of Sciences (2021). DOI: 10.1073/pnas.2101144118
Journal information: Proceedings of the National Academy of Sciences
Provided by Kanazawa University