Studying chromosomal rearrangements in yeast reveals potential avenue for cancer therapy
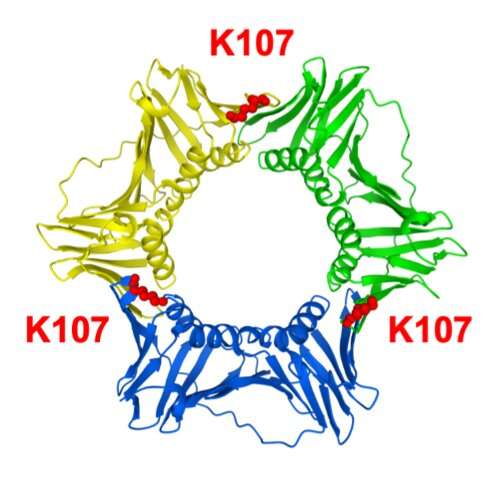
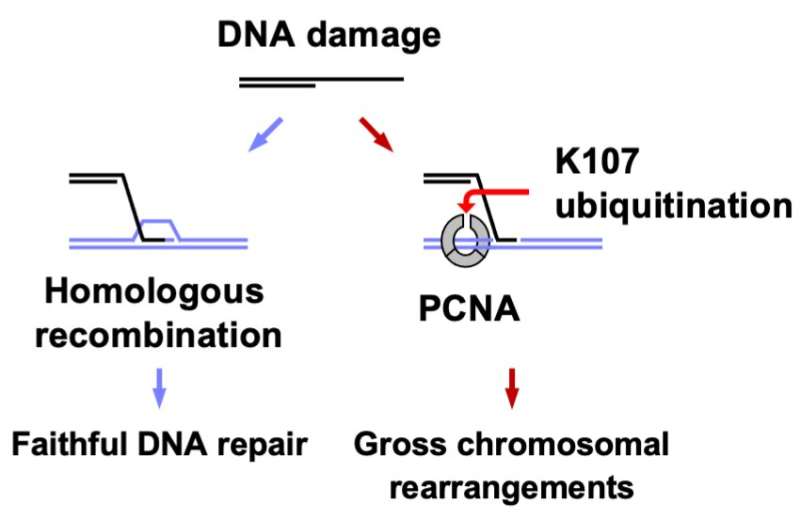
Researchers from Osaka University have found that the attachment of a ubiquitin molecule to a protein called PCNA at the lysine 107 position causes gross chromosomal rearrangements. This lysine is located where two PCNA molecules interact, and the ubiquitin attachment to it may change the ring structure they form. The ubiquitin attachment occurs through the action of Rad8 (a ubiquitin ligase) and Mms2-Ubc4 (a ubiquitin conjugating enzyme). This implies that inhibiting the human equivalent of this ubiquitination could prevent cancer.
Gross chromosomal rearrangements—where portions of the genome become moved, deleted, or inverted—can lead to cell death and diseases such as cancer in complex multicellular organisms. However, the details of how exactly these occur remain unknown. Now, studies in a single-celled organism called fission yeast have found evidence for the involvement of a protein called Rad8.
When DNA replicates or repairs itself, three copies of a protein called PCNA bind together and form a ring-like structure surrounding the DNA strand. This ring structure acts like a clamp and slides along the DNA strand. The team showed that Rad8 attaches a small molecule called ubiquitin to this PCNA protein at the amino acid in the 107th position. This amino acid is a lysine molecule, termed "lysine 107," located at the interface between the different PCNA molecules.
Ubiquitin attachment is a common biological process serving a variety of functions. "In this case, the attachment of ubiquitin at lysine 107 weakens the interactions between PCNA molecules and changes the structure of the ring of proteins," says lead author of the paper Jie Su. This then alters how PCNA functions and leads to the formation of gross chromosomal rearrangements instead of accurate DNA repair.
Rad8 is a ubiquitin ligase, the molecule that is responsible for attaching a ubiquitin to a particular place on another protein. "We found that Rad8 works together with another protein called Mms2-Ubc4, a ubiquitin conjugating enzyme," says Takuro Nakagawa, senior author. "Mms2-Ubc4 brings in the ubiquitin molecule, and Rad8 then transfers the ubiquitin to lysine 107 of PCNA." The combined action of Rad8 and Mms2-Ubc4 is therefore responsible for causing gross chromosomal rearrangements.
But how does this information on a single-celled organism like yeast relate to cancer in complex organisms such as humans? Not much is known about HLTF, the human equivalent to Rad8, but it is seen to be activated and upregulated in cancer. Inhibition of HLTF, or inhibiting the attachment of ubiquitin to PCNA at the human equivalent of the lysine 107 position, could therefore be a very promising new strategy for cancer therapies.
The article, "Fission yeast Rad8/HLTF facilitates Rad52-dependent chromosomal rearrangements through PCNA lysine 107 ubiquitination," was published in PLOS Genetics.
More information: PLOS Genetics (2021). DOI: 10.1371/journal.pgen.1009671
Journal information: PLoS Genetics
Provided by Osaka University