Scientists reveal novel double-roaming mechanism in chemical reaction

Roaming, a novel mechanism in reaction dynamics, describes an unusual pathway that is quite different from the conventional minimum-energy path. It is facilitated by the initial frustrated dissociation to form radical products, and then the meandering of the incipient radicals, ultimately leading to intramolecular abstraction and to the products.
Recently, a group led by Prof. Fu Bina and Prof. Zhang Donghui from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences, in collaboration with Prof. Han Yongchang from Dalian University of Technology, discovered a novel double-roaming mechanism in a combustion reaction.
This work was published in the Journal of Physical Chemistry Letters.
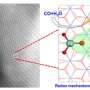
The researchers developed a new, global, full-dimensional potential energy surface (PES) to study the full-dimensional dynamics of the H+HCCH reaction.
They proposed two intriguing and different roaming pathways, namely acetylene-facilitated roaming and vinylidene-facilitated roaming for the H + C2H2 → H2 + C2H reaction.
In the acetylene-facilitated roaming, the frustrated acetylene + H dissociated from the initially formed C2H3 intermediate, and the detachment of the incoming H atom picked up another H atom from acetylene.
In the vinylidene-facilitated roaming, the C2H3 intermediate first underwent the migration of H atom to another carbon atom, and the incoming H atom roamed and found a favorable orientation to abstract the H atom from vinylidene, which was the eventually frustrated vinylidene + H dissociation.
The "double-roaming" pathways accounted for roughly 95% of the total cross section to the H2 + C2H products at the collision energy of 70 kcal/mol. Both roaming pathways produced hot C2H internal energy, while the direct abstraction pathway via the conventional transition state produced cold C2H internal energy.
More information: Yan-Lin Fu et al, Double-Roaming Dynamics in the H + C2H2 → H2 + C2H Reaction: Acetylene-Facilitated Roaming and Vinylidene-Facilitated Roaming, The Journal of Physical Chemistry Letters (2021). DOI: 10.1021/acs.jpclett.1c01045
Journal information: Journal of Physical Chemistry Letters
Provided by Chinese Academy of Sciences