Study reveals peculiar mechanism of radical addition-elimination
For the first time, researchers have shown that a dissociation pathway, or mechanism for breaking chemical bonds, called roaming radical dynamics is a possibility for not just simple, single molecule reactions but more complex, multiple molecule, or bimolecular, reactions. A combination of imaging experiments and high-level quantum chemical calculations allowed for this dissociation pathway in bimolecular reactions to be disentangled both directly and indirectly.
Determining that this mechanism of roaming excursions occur in more complex reactions suggests that it plays a more central role for chemical reactions. This realization should enable more accurate modeling of reactions that govern combustion, atmospheric, and interstellar chemistry.
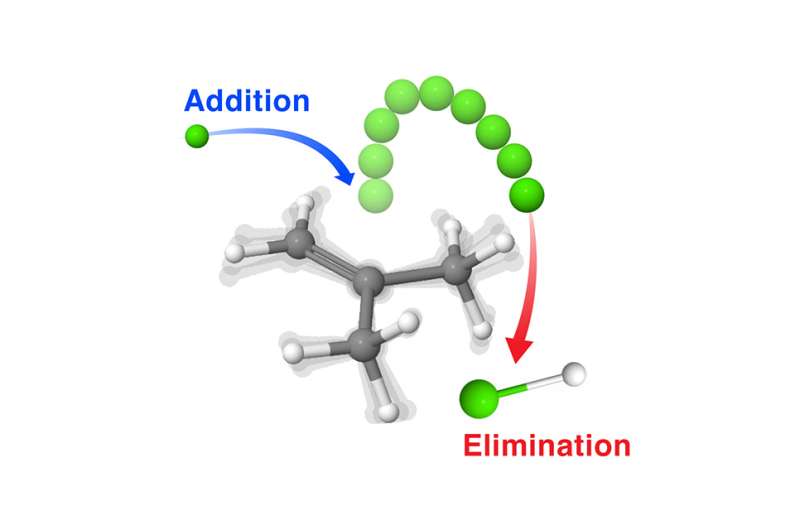
Previously, it was thought that molecules dissociated by two paths – either by breaking bonds between the molecule and creating a pair of smaller molecules or by stretching the bond until it breaks resulting in a pair of fragments called radicals, highly reactive species that are electrically neutral and have unpaired electrons. Recently, a third complex dissociation pathway called "roaming" was revealed where the chemical process starts off by the stretching of a single bond but results in the formation of molecular products instead of radicals. Initial experiments discovering these roaming dynamics looked specifically at reactions where a single molecule rearranges itself to produce one or more products. The question remained whether roaming radical dynamics play a role when a single reaction product arises from two or more distinct starting molecules.
These bimolecular reactions often compete with direct pathways to form the same products, as seen in the gas-phase for reactions of chlorine atoms with unsaturated hydrocarbons. Using crossed-beam studies to provide insight into the molecular forces acting at the exact moment of chemical reactions, researchers studied the scattering distributions when beams of chlorine atoms collide with beams of butane molecules. The team found both direct and indirect pathways with different energy dependence to form the eliminated hydrochloric acid (HCl) product. Using high-level theoretical calculations to understand the reaction mechanisms of the system revealed that to decompose, the energized adducts must undergo near-dissociation back to the starting reactants before eliminating HCl at the adjacent saturated sites. This is a clear example of roaming radical dynamics showing that they play a central role in a broad class of bimolecular reactions and may be important for a wide range of environmental and industrially relevant chemical reactions.
More information: "Roaming dynamics in radical addition-elimination reactions." Nature Communications 5, 4064 (2014). DOI: 10.1038/ncomms5064
Journal information: Nature Communications
Provided by US Department of Energy