July 13, 2020 feature
Long-term heat-storage ceramics absorbing thermal energy from hot water
Approximately seventy percent of the thermal energy generated in thermal and nuclear power plants is lost as waste heat, with a temperature below the boiling point of water. In a recent report on Science Advances, Yoshitaka Nakamura and a research team in chemistry, materials, and technology in Japan developed a long-term heat storage material to absorb heat energy at warm temperatures ranging from 38 degrees C (311 K) to 67 degrees C (340 K). They composed the unique series of materials using scandium-substituted lambda-trititanium-pentoxide (λ-ScxTi3−xO5). The construct accumulated heat energy from hot water and released the accumulated heat energy upon the application of pressure. The new material has the potential to accumulate the heat energy of hot water generated in nuclear and thermal power plants, then recycle the stored heat energy on demand based on external pressures. The material is also applicable to recycle waste heat in industrial factories and automobiles.
First-principles calculations of formation energy and determining the crystal structure
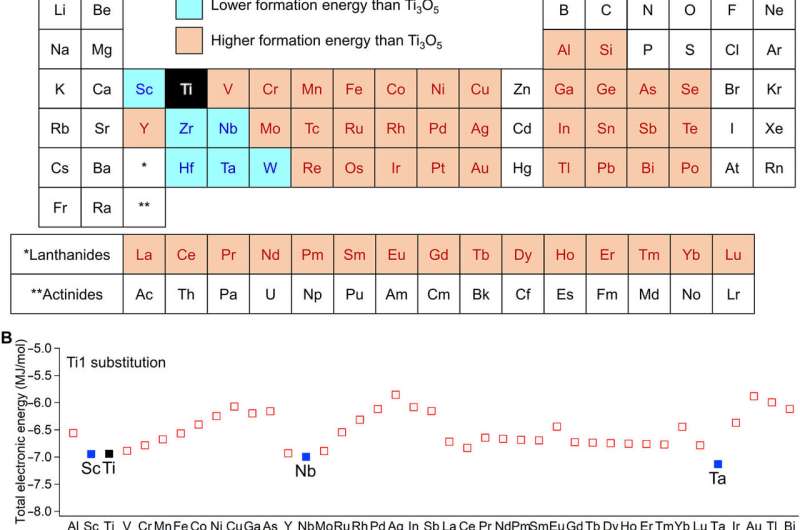
The team used the metal-substituted lambda-trititanium-pentoxide (λ-MxTi3O5) during the experiments to realize heat storage materials that can absorb low-temperature waste heat and exhibit photo- and pressure-induced phase transitions. Scientists had previously reported several types of metal-substituted λ-Ti3O5. In this work, Nakamura et al. surveyed 54 elements as metal cations suited for metal substitution of the Ti ion in λ-Ti3O5. Of these, only six had a stabilizing effect including scandium, niobium, tantalum, zirconium, hafnium and tungsten. The team then reported the synthesis of the crystal structure and heat storage properties of the Sc-substituted λ-Ti3O5 in the λ phase.
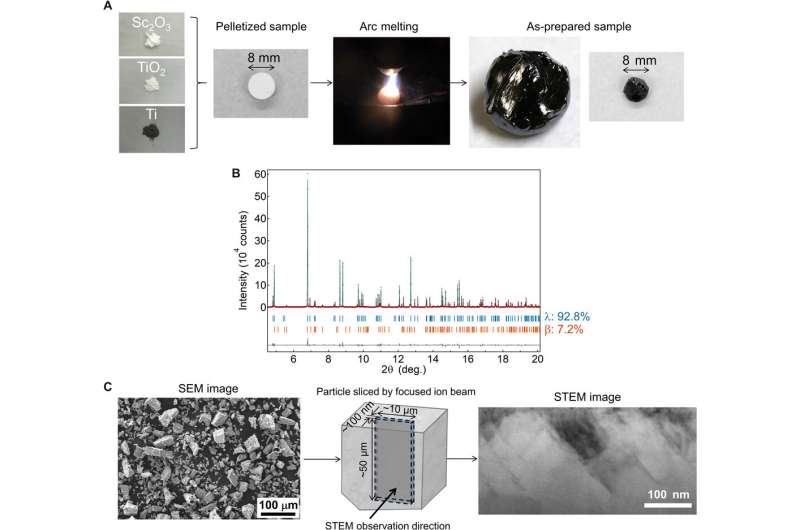
To synthesize the Sc-substituted compound, Nakamura et al. used an arc-melting technique in an Argon atmosphere. During the process, they mixed precursors of Sc2O3, TiO2 and Ti powders to prepare an 8-mm pellet of the mixture shaped into a spherical ball. Then using X-ray fluorescence (XRF) measurements they determined the formula of the sample (Sc0.9Ti2.91O5) and performed synchrotron X-ray diffraction (SXRD) to determine the crystal structure. The results corresponded to the crystal structure of λ-Ti3O5 with 0.4 percent expansion after metal substitution. Using scanning transmission electron microscopy (STEM) images the team obtained stripe-like domains in the compound.
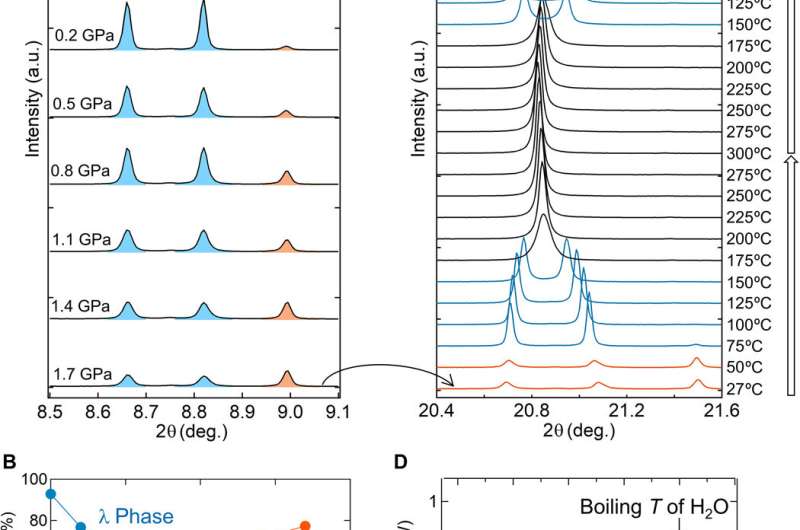
Pressure-induced phase transitions, heat-storage properties, and long-term heat storage mechanisms
The team next measured the pressure-induced phase transition using SXRD (synchrotron X-ray diffraction) after compressing the samples with a hydraulic press. When the pressure increased, the λ-phase fraction of the sample decreased, and the β-phase fraction increased in a reversible process. They measured the heat absorption mass of the sample after the pressure-induced phase transition (λ- to β-phase) using differential scanning calorimetry (DSC). They noted the heat absorption of the material with an absorption peak at 67 degrees C and observed repeated pressure- and heat-induced phase transitions. During phase transitions from the β-phase to the λ-phase, the heat storage temperature remarkably reduced from a previously recorded value of 197 degrees C to 67 degrees C in the present work.
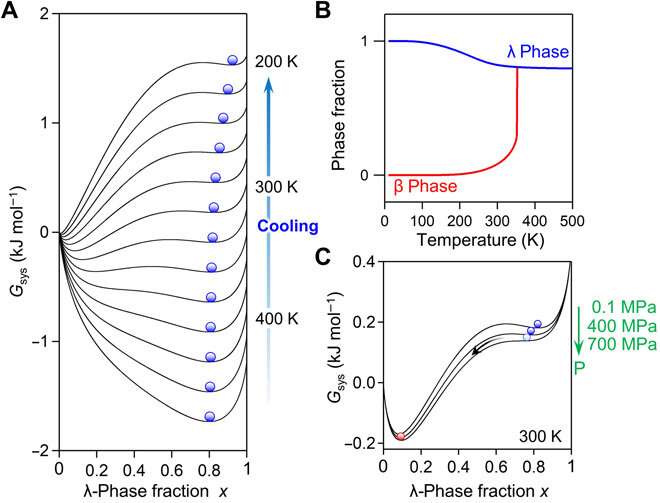
Previous reports on λ-Ti3O5 also credited the reversible phase transition between the λ-phase and β-phase by pressure and heat to the energy barrier between the two phases, which originates from the elastic interaction within the material. To understand the mechanisms of long-term heat storage and low-pressure induced heat energy release in this setup, Nakamura et al. calculated the Gibbs free energy of the system. For this, they used a thermodynamic model based on the Slichter and Drickamer (SD model). During the process of phase transition, the scientists could maintain the λ-phase for a prolonged time since the energy barrier between the two phases prevented the immediate transfer of the λ-phase in to the β-phase. The resulting Sc0.9Ti2.91O5 prepared in the work showed good stability and could be maintained perfectly for about eight months to one year from the XRD measurement.
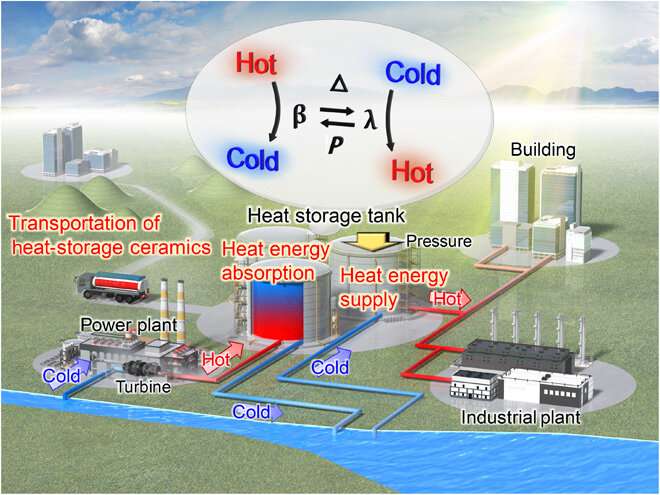
Proof of concept
The scientists investigated the heat-storage system with Sc-substituted λ-Ti3O5 in a practical setting by pumping cooling water for a turbine in a power plant from a river or sea. As the water passed through the turbine, its temperature increased due to heat exchange, transferring the energy of hot water to Sc-substituted λ-Ti3O5 materials used in the tanks. Meanwhile, water with a reduced thermal energy returned to the river or the sea. Energy stored in the Sc-substituted λ-Ti3O5 could be released in the form of thermal energy by applying pressure for energy use on demand. Nakamura et al. envision supplying the stored thermal energy to building or industrial plants that are close to power plants without using electricity.
In this way, Yoshitaka Nakamura and colleagues demonstrated heat storage ceramics based on Sc-substituted λ-Ti3O5, which absorbed heat from water. Based on first-principles calculations, they synthesized Sc-substituted λ-Ti3O5 ceramics with a heat absorption below 100 degrees C. The heat absorption material recovered thermal energy from cooling water in power plant turbines and could be easily controlled by changing the Sc content in Ti3O5 relative to the application of interest. In addition to its functions in electric power plants, the scientists propose using the materials for heat storage functions by collecting waste heat from regular devices such as mobile phones, transport vehicles, from factories and electronic devices.
More information: Yoshitaka Nakamura et al. Long-term heat-storage ceramics absorbing thermal energy from hot water, Science Advances (2020). DOI: 10.1126/sciadv.aaz5264
David Lindley. The energy should always work twice, Nature (2009). DOI: 10.1038/458138a
Michelle T. H. van Vliet et al. Vulnerability of US and European electricity supply to climate change, Nature Climate Change (2012). DOI: 10.1038/nclimate1546
Journal information: Science Advances , Nature , Nature Climate Change
Provided by Science X Network
© 2020 Science X Network