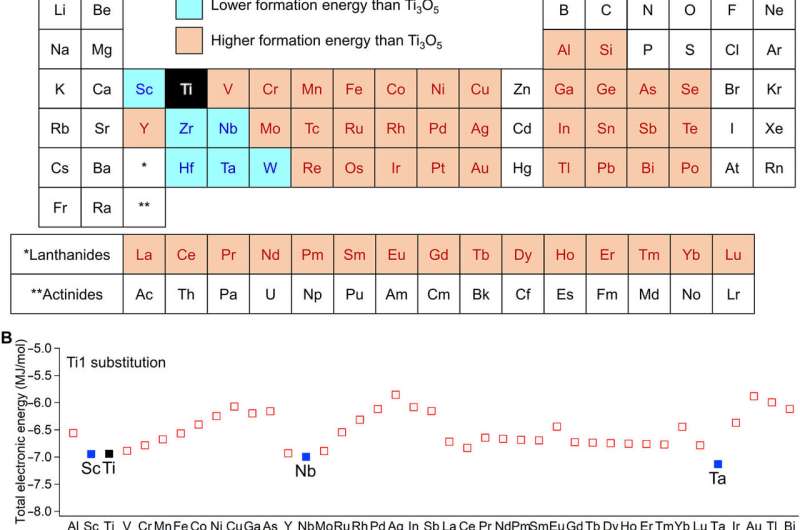
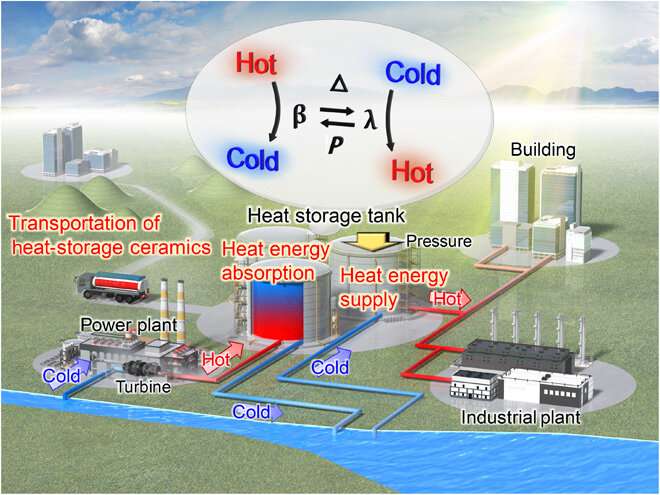
First-principles calculations of formation energies. (A) Periodic table colored by the total electronic energies of λ-Ti3O5 with an elemental substitution. Blue elements are those where substituted λ-Ti3O5 shows a lower formation energy than that of pure λ-Ti3O5. Orange elements are those where substituted λ-Ti3O5 shows a higher formation energy. (B) Calculated total electronic energies of λ-AxTi3−xO5 (A, trivalent elements) and (C) λ-BxTi3-xO5 (B, tetravalent elements) in order of atomic number. One of three Ti sites in λ-Ti3O5 is substituted by a colored element for the first-principles calculations. Element A in λ-AxTi3−xO5 substitutes into the Ti1 site. Element B in λ-BxTi3-xO5 substitutes into the Ti2 site. Blue and orange squares represent that elemental substituted λ-Ti3O5 shows a lower formation and a higher formation energy, respectively. Black square denotes pure λ-Ti3O5. Credit: Science Advances, doi: 10.1126/sciadv.aaz5264
Approximately seventy percent of the thermal energy generated in thermal and nuclear power plants is lost as waste heat, with a temperature below the boiling point of water. In a recent report on Science Advances, Yoshitaka Nakamura and a research team in chemistry, materials, and technology in Japan developed a long-term heat storage material to absorb heat energy at warm temperatures ranging from 38 degrees C (311 K) to 67 degrees C (340 K). They composed the unique series of materials using scandium-substituted lambda-trititanium-pentoxide (λ-ScxTi3−xO5). The construct accumulated heat energy from hot water and released the accumulated heat energy upon the application of pressure. The new material has the potential to accumulate the heat energy of hot water generated in nuclear and thermal power plants, then recycle the stored heat energy on demand based on external pressures. The material is also applicable to recycle waste heat in industrial factories and automobiles.
First-principles calculations of formation energy and determining the crystal structure
The team used the metal-substituted lambda-trititanium-pentoxide (λ-MxTi3O5) during the experiments to realize heat storage materials that can absorb low-temperature waste heat and exhibit photo- and pressure-induced phase transitions. Scientists had previously reported several types of metal-substituted λ-Ti3O5. In this work, Nakamura et al. surveyed 54 elements as metal cations suited for metal substitution of the Ti ion in λ-Ti3O5. Of these, only six had a stabilizing effect including scandium, niobium, tantalum, zirconium, hafnium and tungsten. The team then reported the synthesis of the crystal structure and heat storage properties of the Sc-substituted λ-Ti3O5 in the λ phase.
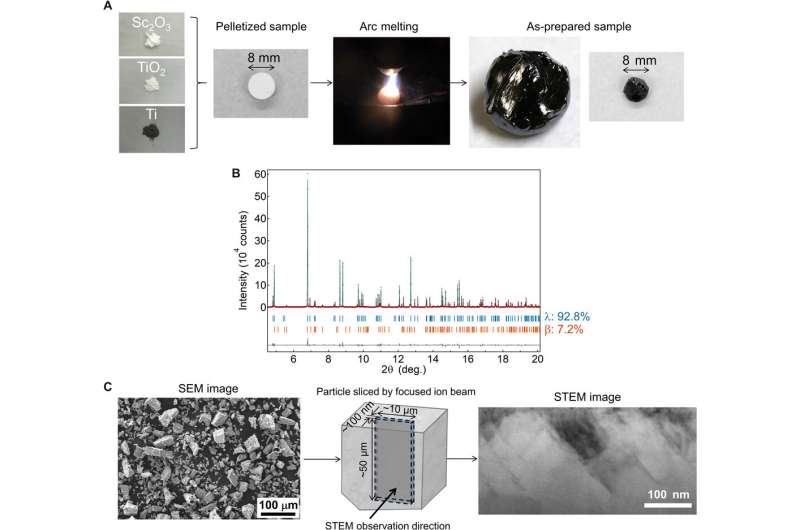
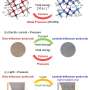
Synthesis, crystal structure, and morphology of λ-Sc0.09Ti2.91O5. (A) λ-Sc0.09Ti2.91O5 sample synthesis. Pelletized mixture powder of Sc2O3, TiO2, and Ti metal with a diameter of 8 mm is prepared, melted, and rapidly cooled in an arc-melting process. After the melting process, the solidified (as-prepared) sample is milled by hand. Photo credit: Yoshitaka Nakamura, Panasonic Corporation. (B) Synchrotron x-ray diffraction (SXRD) pattern of the as-prepared Sc0.09Ti2.91O5 sample collected at room temperature with λ = 0.420111 Å. Upper blue and lower orange bars represent the calculated positions of the Bragg reflections of λ-Sc0.09Ti2.91O5 and β-Sc0.09Ti2.91O5. (C) Scanning electron microscopy (SEM) image of the powdered sample shows a grain size below 100 μm. Particle from the powdered sample is sliced by a focused ion beam. STEM image shows stripe-like domains with a size of about 100 nm × 200 nm. Scale bars show 100 μm in the SEM image and 100 nm in the STEM image. Credit: Science Advances, doi: 10.1126/sciadv.aaz5264
To synthesize the Sc-substituted compound, Nakamura et al. used an arc-melting technique in an Argon atmosphere. During the process, they mixed precursors of Sc2O3, TiO2 and Ti powders to prepare an 8-mm pellet of the mixture shaped into a spherical ball. Then using X-ray fluorescence (XRF) measurements they determined the formula of the sample (Sc0.9Ti2.91O5) and performed synchrotron X-ray diffraction (SXRD) to determine the crystal structure. The results corresponded to the crystal structure of λ-Ti3O5 with 0.4 percent expansion after metal substitution. Using scanning transmission electron microscopy (STEM) images the team obtained stripe-like domains in the compound.
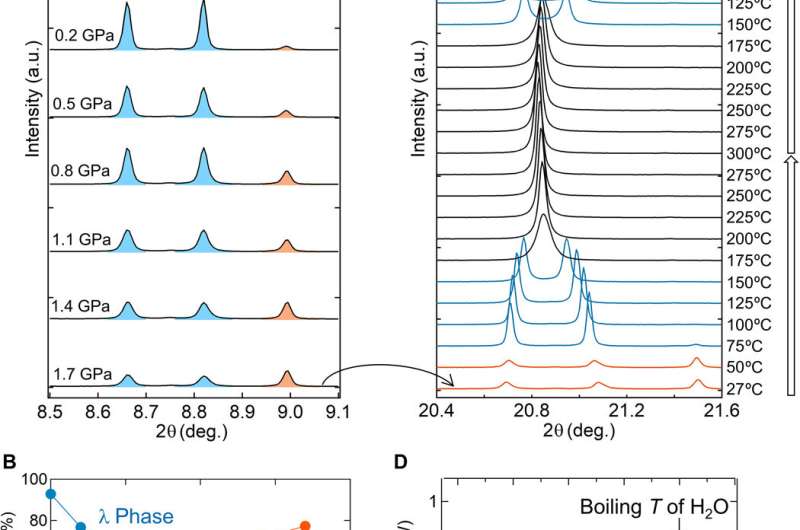
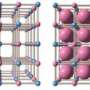
Pressure-induced phase transition and heat-storage process. (A) SXRD patterns of Sc0.09Ti2.91O5 measured at room temperature and ambient pressure after compression between 0.2 and 1.7 GPa with a hydraulic press (λ = 0.420111 Å). As the pressure increases, the λ-(20-3) and λ-(203) peaks (blue) decrease and the β-(20-3) peak (orange) increases, indicating a pressure-induced phase transition. a.u., arbitrary units. (B) Pressure dependence of the phase fractions of Sc0.09Ti2.91O5 calculated from the SXRD patterns in (A). Crossover pressure (phase transition pressure) occurs at 670 MPa. (C) SXRD patterns of Sc0.09Ti2.91O5 measured between 27°C (300 K) and 300°C (573 K; λ = 0.999255 Å). The λ and β peaks are constant until 50°C (323 K; orange), and then the β phase decreases and the λ phase increases at 75°C (348 K; blue). The λ phase transforms into the α phase above 175°C (448 K; black) but is restored upon cooling. (D) DSC chart of Sc0.09Ti2.91O5 shows an endothermic reaction at 67°C (340 K). Samples are compressed at 1.7 GPa before the variable temperature SXRD and DSC chart measurements. Credit: Science Advances, doi: 10.1126/sciadv.aaz5264
Pressure-induced phase transitions, heat-storage properties, and long-term heat storage mechanisms
The team next measured the pressure-induced phase transition using SXRD (synchrotron X-ray diffraction) after compressing the samples with a hydraulic press. When the pressure increased, the λ-phase fraction of the sample decreased, and the β-phase fraction increased in a reversible process. They measured the heat absorption mass of the sample after the pressure-induced phase transition (λ- to β-phase) using differential scanning calorimetry (DSC). They noted the heat absorption of the material with an absorption peak at 67 degrees C and observed repeated pressure- and heat-induced phase transitions. During phase transitions from the β-phase to the λ-phase, the heat storage temperature remarkably reduced from a previously recorded value of 197 degrees C to 67 degrees C in the present work.
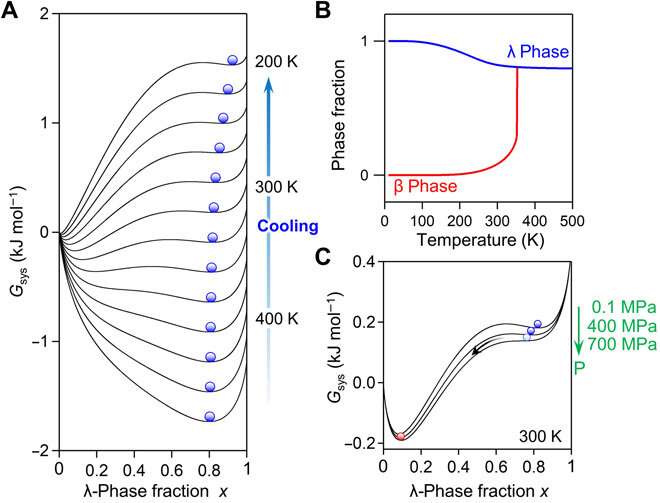
Mechanism of long-term heat storage and pressure-induced phase transition. (A) Gibbs free energy (Gsys) versus λ phase fraction (x) curves from 420 to 200 K with a 20 K interval, calculated by the SD model. Blue spheres indicate the thermal population of the λ phase. (B) Temperature dependence of the calculated λ phase (blue) and β phase (red) fractions. (C) Gsys versus x under ambient pressures of 0.1, 400, and 700 MPa at 300 K. Credit: Science Advances, doi: 10.1126/sciadv.aaz5264
Previous reports on λ-Ti3O5 also credited the reversible phase transition between the λ-phase and β-phase by pressure and heat to the energy barrier between the two phases, which originates from the elastic interaction within the material. To understand the mechanisms of long-term heat storage and low-pressure induced heat energy release in this setup, Nakamura et al. calculated the Gibbs free energy of the system. For this, they used a thermodynamic model based on the Slichter and Drickamer (SD model). During the process of phase transition, the scientists could maintain the λ-phase for a prolonged time since the energy barrier between the two phases prevented the immediate transfer of the λ-phase in to the β-phase. The resulting Sc0.9Ti2.91O5 prepared in the work showed good stability and could be maintained perfectly for about eight months to one year from the XRD measurement.
Application of Sc-substituted λ-Ti3O5 for power plants. Schematic illustration of a heat energy recycling system using Sc-substituted λ-Ti3O5 heat-storage ceramics. Cooling water for a turbine in a power plant is pumped from a river or sea. Water becomes hot after heat exchange through the turbine. This hot water energy is stored in tanks containing Sc-substituted λ-Ti3O5 heat-storage ceramics. Water with a reduced heat energy returns to the river or the sea, mitigating the rise of the sea temperature. Energy-stored Sc-substituted λ-Ti3O5 heat-storage ceramics can supply heat energy to buildings or industrial plants by applying pressure. Furthermore, the energy-stored ceramics can be transported to distant locations by a truck. Credit: Science Advances, doi: 10.1126/sciadv.aaz5264
Proof of concept
The scientists investigated the heat-storage system with Sc-substituted λ-Ti3O5 in a practical setting by pumping cooling water for a turbine in a power plant from a river or sea. As the water passed through the turbine, its temperature increased due to heat exchange, transferring the energy of hot water to Sc-substituted λ-Ti3O5 materials used in the tanks. Meanwhile, water with a reduced thermal energy returned to the river or the sea. Energy stored in the Sc-substituted λ-Ti3O5 could be released in the form of thermal energy by applying pressure for energy use on demand. Nakamura et al. envision supplying the stored thermal energy to building or industrial plants that are close to power plants without using electricity.
In this way, Yoshitaka Nakamura and colleagues demonstrated heat storage ceramics based on Sc-substituted λ-Ti3O5, which absorbed heat from water. Based on first-principles calculations, they synthesized Sc-substituted λ-Ti3O5 ceramics with a heat absorption below 100 degrees C. The heat absorption material recovered thermal energy from cooling water in power plant turbines and could be easily controlled by changing the Sc content in Ti3O5 relative to the application of interest. In addition to its functions in electric power plants, the scientists propose using the materials for heat storage functions by collecting waste heat from regular devices such as mobile phones, transport vehicles, from factories and electronic devices.
More information: Yoshitaka Nakamura et al. Long-term heat-storage ceramics absorbing thermal energy from hot water, Science Advances (2020). DOI: 10.1126/sciadv.aaz5264
David Lindley. The energy should always work twice, Nature (2009). DOI: 10.1038/458138a
Michelle T. H. van Vliet et al. Vulnerability of US and European electricity supply to climate change, Nature Climate Change (2012). DOI: 10.1038/nclimate1546
Journal information: Science Advances , Nature , Nature Climate Change
Provided by Science X Network
© 2020 Science X Network