Interfacial chemistry improves rechargeability of Zn batteries
With strong interest in environmentally benign and efficient resource utilization, green and safe battery systems are in demand, and improving rechargeability is a goal. Since the surface chemistry of the solid-electrolyte interphase (SEI) is a critical factor governing the cycling life of rechargeable batteries, it is a key research focus.
Zn batteries (ZBs) are characterized by low cost, superior volumetric energy output and cost-effective raw materials, making them a promising candidate to meet the demand for rechargeable batteries. However, some characteristics of the Zn-electrolyte interface restrict the development of rechargeable ZBs and their application.
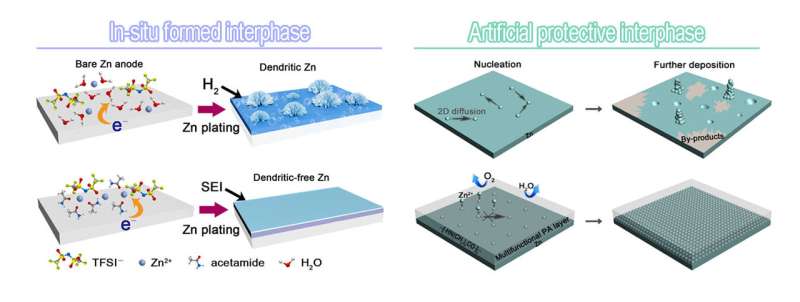
Prof. Cui Guanglei's group from the Qingdao Institute of Bioenergy and Bioprocess Technology of the Chinese Academy of Sciences has proposed new concepts concerning in situ formed and artificial SEIs as a means of fundamentally modulating the electrochemical characteristics of Zn.
By manipulating the decomposition of a eutectic liquid with a peculiar anion-associated cation solvation structure, the researchers observed zinc fluoride-rich organic/inorganic SEI on a Zn anode for the first time.
A combination of experimental and modeling investigations revealed that the presence of anion-complexing Zn species with markedly lowered decomposition energies contributed to the in-situ formation of the interphase.
"The protective interphase enables reversible and dendrite-free Zn plating/stripping even at high areal capacities. This is due to the fast ion migration coupled with high mechanical strength," said Prof. Cui.
With this interfacial design, the assembled Zn batteries exhibited excellent cycling stability with negligible capacity loss at both low and high rates.
In addition, coating the Zn surface with an artificial protective polyamide layer is easy to implement. The polyamide layer has all the desirable characteristics for supporting highly reversible Zn chemistry with enhanced cycling performance of Zn anodes at neutral pH, even at a high depth of discharge.
The study offers new insights into the rational regulation of Zn anodes and provides an unprecedented avenue for tackling the dilemmas raised by the intrinsic properties of multivalent metal anodes.
More information: Huayu Qiu et al, Zinc anode-compatible in-situ solid electrolyte interphase via cation solvation modulation, Nature Communications (2019). DOI: 10.1038/s41467-019-13436-3
Journal information: Nature Communications
Provided by Chinese Academy of Sciences