A new method for conformal deposition of manganese oxide on high aspect-ratio substrates
Manganese oxides have numerous applications in batteries, supercapacitors, microelectronics and (electro)catalysis—all of which can greatly benefit from conformally deposited MnO2 on high aspect ratio structures, e.g. 3-D battery current collectors, or high surface area catalytic supports.
Recently published in ACS Chemistry of Materials, researchers from imec, KU Leuven and Ghent University developed a cheap and fast method for depositing conformal thin films of MnO2 on nanostructured substrates with close-to-a-monolayer precision, competing with the state-of-the-art atomic layer deposition (ALD).
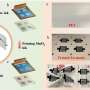
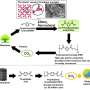
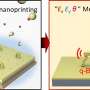
The new method was inspired by the first-grade high-school demonstration of a redox reaction, where aqueous potassium permanganate (KMnO4) is reduced by an alcohol (e.g. ethanol) at a neutral pH, forming solid MnO2 in the bulk of the solution. In the new method, the quantity of as-formed MnO2 was limited to a monolayer by using aqueous propargyl alcohol—an unsaturated alcohol which can strongly chemisorb on various substrates, allowing reduction of its amount to a monolayer for the subsequent reaction with KMnO4. Thus, the method consists of repeating cycles of surface-limited adsorption of propargyl alcohol and its subsequent oxidation with aqueous potassium permanganate, forming a controllable amount of MnO2 on the substrate in each cycle.
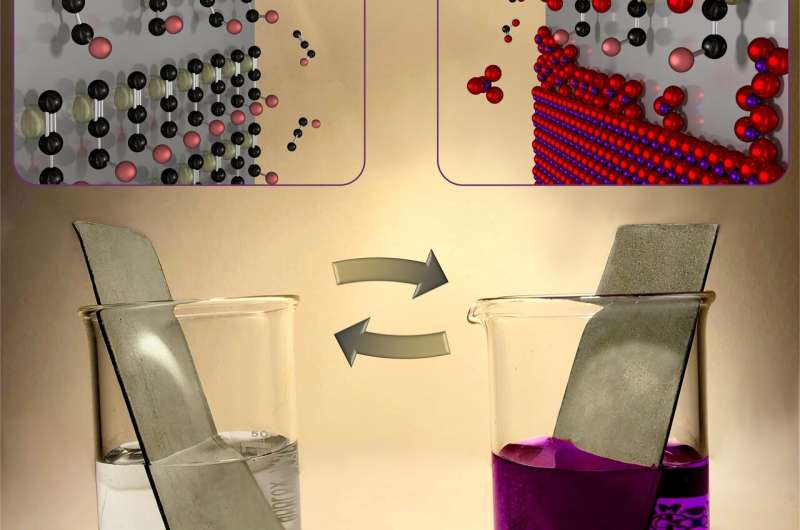
Because the amount of manganese oxide formed in each cycle is limited by the monolayer quantity of the adsorbed alcohol, the growth exhibits the self-limiting characteristics of atomic layer deposition (ALD). This state-of-the-art technique is based on a cyclic reaction of gaseous precursors on a surface, and typically ensures the highest conformality of the coating and sub-monolayer thickness control, at an expense of a very low deposition rate, need for elevated temperatures, expensive precursors and complex, thermally isolated gas-tight reactors.
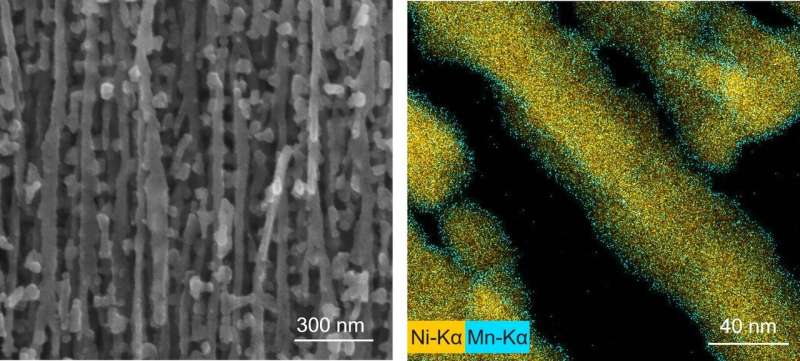
Contrary to the typical ALD, the new redox layer deposition (RLD) is performed in air, at room temperature, using common and cheap chemicals and simple glassware—literally, two beakers. This greatly reduces the costs and complexity of the deposition, making it accessible to virtually any laboratory or manufacturing plant. The method also shows at least 4x higher growth-per-cycle and is at least 1.5x faster than the known ALD process of MnO2 thanks to the high adsorption density of the alcohol molecules and MnO4- ions on the substrates. The RLD method was also successfully used to coat complex 3-D-interconnected Ni nanowires with thin MnO2, which could not be performed with the typical thermal ALD.
This work is the first demonstration of an ALD-like growth of a metal oxide performed entirely in aqueous phase and open air. This is an important differentiator from the few previously reported liquid-phase ALD processes of some metal oxides (e.g. MnOx, TiO2 or MgO), which all utilized water-sensitive precursors dissolved in organic solvents and, thus, required anhydrous conditions and neutral gas environment of a glovebox or a Schlenk line. Although currently limited to substrates made of transition metals (e.g. Ni, Ti, Pt) and their oxides (e.g. TiO2), the range of compatible substrates might be increased in the future to e.g. Al2O3 or SiO2, by choosing appropriate organic adsorbates. Also, the RLD method could be tested for depositing other oxides than MnO2, by using different metal complexes that form insoluble products during redox reaction.
Overall, thanks to its simplicity, the conformal deposition of MnO2 can be easily upscaled and thus exploited for its numerous (electro) chemical applications.
More information: Stanislaw P. Zankowski et al. Redox Layer Deposition of Thin Films of MnO2 on Nanostructured Substrates from Aqueous Solutions, Chemistry of Materials (2019). DOI: 10.1021/acs.chemmater.9b01219
Journal information: Chemistry of Materials
Provided by IMEC