Can't get thinner than this: synthesis of atomically flat boron sheets
Since its rediscovery and characterization in 2004, graphene has been the focus of countless research efforts across multiple fields. It is a versatile material consisting of a two-dimensional (2-D) carbon network, a thin sheet of carbon that has a thickness of one atom. Graphene is not only stronger than the strongest steels, but also has a myriad of interesting chemical, electronic, and mechanical characteristics that has left scientists wondering if similar 2-D networks of other materials could have such useful properties.
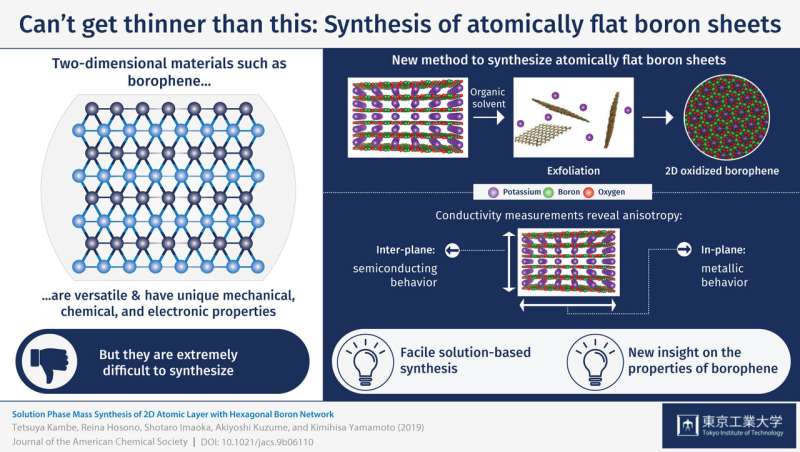
One novel 2-D material that was recently reported is borophene, an analogue of graphene consisting of boron atoms instead of carbon atoms. However, as one would expect for 2-D sheets of any material, the synthesis of borophene has proved to be challenging. Researchers either require the use of a substrate to make borophene more stable or coupling boron with hydroxyl groups (OH-), which prevents atomic flatness.
In a recent study conducted at Tokyo Institute of Technology, a research team including Tetsuya Kambe, Akiyoshi Kuzume and Kimihisa Yamamoto synthesized atomically flat oxidized borophene sheets through a simple solution-based method. First, they synthesized stacked layers of borophene oxide through a fairly simple process using a potassium borohydride salt (KBH4). An X-ray analysis revealed the 2-D-layered structure of the material, in which layers of boron atoms forming a hexagonal 2-D network with oxygen atoms as bridges were intercalated with layers containing potassium atoms. Then, the subsequent step was to find a way to exfoliate atomically thin layers of the borophene oxide network. The researchers achieved this by putting the material in dimethylformamide, which is a commonly used organic solvent. Various types of measurements were carried out to verify the structure of the exfoliated sheets, including electron microscopy, spectroscopy, and atomic force microscopy. The results confirmed that the proposed method was effective for producing the desired atomically flat oxidized borophene sheets.
Finally, the researchers performed resistivity measurements to analyze the conducting properties of stacked borophene sheets and found an interesting characteristic referred to as anisotropy. This means that the sheets exhibited different types of conductivity depending on the direction of the current flow. The material behaved like a semiconductor in the inter-plane direction, whereas it exhibited metal-like behavior in the in-plane direction of the boron network. The mechanisms behind these two types of conducting behaviors were elucidated, as well. "It is important to note that our boron sheets can be handled easily at ambient conditions," said Dr. Kambe, indicating that this pioneering research could lead to practical applications for borophene.
Finding facile methods for the synthesis of borophene and borophene-based compounds is crucial to conducting further research on this interesting material and its potential uses. "Like graphene, borophene is expected to have unique properties, including extraordinary mechanical characteristics and metallic behavior that could be exploited in a variety of fields," said Dr. Kambe. Hopefully, future findings and developments on 2-D materials will enable researchers to employ their exotic properties and tailor them to suit specific needs.
More information: Tetsuya Kambe et al, Solution Phase Mass Synthesis of 2D Atomic Layer with Hexagonal Boron Network, Journal of the American Chemical Society (2019). DOI: 10.1021/jacs.9b06110
Journal information: Journal of the American Chemical Society
Provided by Tokyo Institute of Technology