Gold soaks up boron, spits out borophene
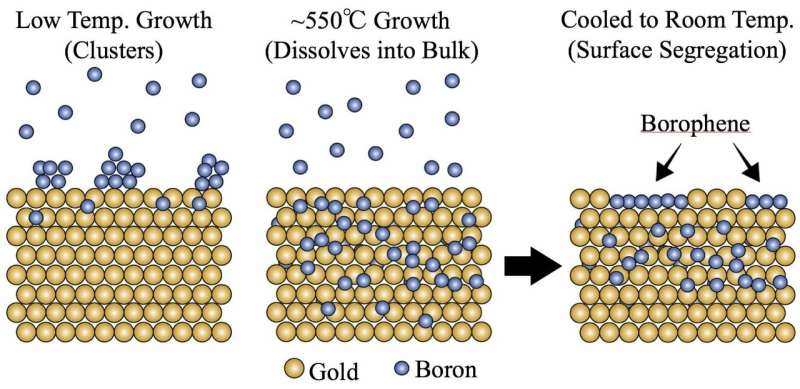
In the heat of a furnace, boron atoms happily dive into a bath of gold. And when things get cool, they resurface as coveted borophene.
The discovery by scientists from Rice University, Argonne National Laboratory and Northwestern University is a step toward practical applications like wearable or transparent electronics, plasmonic sensors or energy storage for the two-dimensional material with excellent conductivity.
Teams led by Boris Yakobson at Rice, Nathan Guisinger at Argonne and Mark Hersam at Northwestern both formed the theory for and then demonstrated their novel method to grow borophene – the atom-thick form of boron – on a gold surface.
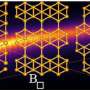
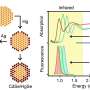
They found that with sufficient heat in a high vacuum, boron atoms streamed into the furnace sink into the gold itself. Upon cooling, the boron atoms reappear and form islands of borophene on the surface.
This is distinct from most other 2-D materials made by feeding gases into a furnace. In standard chemical vapor deposition, the atoms settle onto a substrate and connect with each other. They typically don't disappear into the substrate.
The discovery was described in a paper in the American Chemical Society journal ACS Nano.
The researchers said the metallic borophene islands are about 1 nanometer square, on average, and show evidence of electron confinement, which could make them practical for quantum applications.
Yakobson said trying various substrates could yield new phases of borophene with new properties. "Gold, with a lesser charge transfer and weaker bonding, may yield a layer that's easier to lift off and put to use, although this has not yet been achieved," he said.
Yakobson has a track record with borophene, which cannot be exfoliated from bulk materials like graphene can from graphite. A materials theorist, he predicted in 2013 that it could be made at all. A couple of years later, it was.
He and his colleagues on the new paper, Hersam and Guisinger, had already showed that borophene grown in a particular way on silver becomes wavy, which gives it interesting possibilities for wearable electronics.
"So far, the substrates with demonstrated success for borophene synthesis closely follow theoretical predictions," Yakobson said. Argonne has successfully grown it on silver and copper as well as gold, while the Chinese Academy of Sciences has grown borophene on aluminum.
Now, with their work on gold, they have combined theory and experiments to demonstrate an entirely new mechanism of growth for two-dimensional materials.
"The challenge remains to grow it on an insulating substrate," he said. "That will permit many intriguing experimental tests, from basic transport to plasmons to superconductivity."
The researchers found it took an order of magnitude more boron to grow borophene on gold than it did for silver. That was their first indication that boron was sinking into the gold, which started happening at about 550 degrees Celsius (1,022 degrees Fahrenheit).
Yakobson noted a low number of atoms remain embedded in the gold without forming an alloy, but scientists have seen signs of that phenomenon before. "In graphene growth on common copper, carbon atoms also partially dissolve and diffuse through the foil, without a specific alloy being formed," he said.
More information: Brian Kiraly et al. Borophene Synthesis on Au(111), ACS Nano (2019). DOI: 10.1021/acsnano.8b09339
Journal information: ACS Nano
Provided by Rice University