April 19, 2016 report
New two-dimensional 'borophene' sheet
(Phys.org)—Boron, the neighboring element to carbon on the periodic table, has chemical features that make it an enticing candidate for two-dimensional, conductive, atomically homogenous substrates similar to graphene. Three-dimensional bulk boron is non-metallic and is used in semiconductor chemistry. However, theoretical studies and prior work in trying to isolate two-dimensional boron show that two-dimensional boron should have metallic properties. To date, only one two-dimensional boron allotrope has been identified.
Researchers from the Institute of Physics at the Chinese Academy of Sciences and the Collaborative Innovation Center of Quantum Matter in China have identified two new types of boron sheets that they grew on an Ag(111) surface. The sheets are stable, relatively inert to oxidation, and bind loosely to the silver substrate, making these "borophene" sheets excellent candidates for further research into boron-based electronic devices. This work appears in Nature Chemistry.
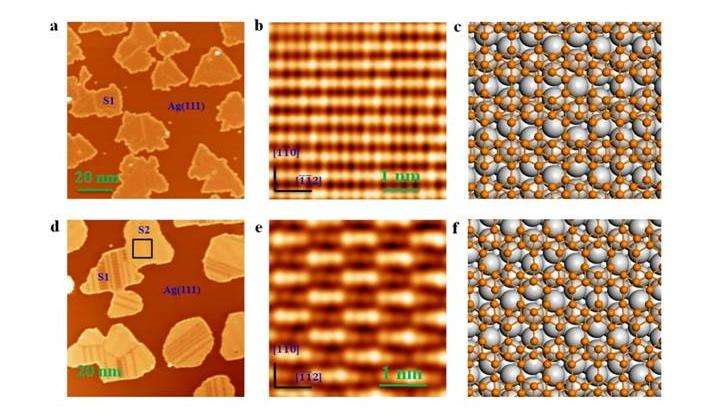
Using molecular beam epitaxy to deposit boron onto a silver substrate, Baojie Feng, Jin Zhang, Qing Zhong, Wenbin Li, Shuai Li, Hui Li, Peng Cheng, Sheng Meng, Lan Chen, and Kehui Wu report the formation of two structurally different boron sheets. Both are comprised of a triangular boron lattice, but the hexagonal holes are arranged differently in the two sheets. These sheets follow theoretical predictions that B36 units should have a triangle lattice with a hexagonal hole in the center.
The boron sheets were grown on a single-crystal of Ag(111) in high vacuum using direct evaporation of pure boron. Scanning tunneling microscopy studies revealed two different phases that were observed as temperature increased. The first phase, labeled S1, was observed at temperatures above 570K and corresponds to theoretical β12 sheets. The second phase, labeled S2, was observed at temperatures above 650K and corresponds to theoretical χ3 sheets. At higher temperatures, most of the S1 phase converts to S2. Both appear as monolayer islands of parallel boronphene stripes with parallel rows or protrusions.
Electron charge analysis confirms the locations of boron atoms on the silver surface and boron atomic density studies provided further evidence for the formation of β12 and χ3 sheets. The atomic density for the S1 phase was found to be 33.6 +/- 2.0 nm-2, which is very close to theoretical β12 value of 34.48nm-2. The boron density did not change for the S2 phase indicative of the χ3 structure, which has a predicted atomic density of 31.3nm-2.
Using XPS studies, Feng, et al. found that the boron sheets are relatively stable, particularly within the borophene islands. The edges of the islands tended to oxidize, while the center remained relatively unchanged. This remained the case even after subjecting the sheets to high concentrations of oxygen gas.
β12 and χ3 sheets were not necessarily the lowest energy borophene sheets that the models had predicted. In an effort to understand why these structures were observed over the predicted energetically minimum state, Feng, et al. conducted energy formation studies and found that interaction with the silver surface makes formation of the β12 and χ3 sheets thermodynamically favorable.
However, even though the silver surface is important for the formation of the sheets, the interaction between the boron sheet and the Ag(111) surface is not very strong. Several factors, including adhesion energy calculations, the distance between the boron sheet and the substrate, and the small charge transfer between the substrate and boron sheet, indicate that the boron sheets are predominantly bound to the surface via the edges. This means that the borophene sheets could be separated from the silver surface, similar to graphene.
Several recent publications have spurned greater interest in these borophene sheets. Theoretical work by researchers from Rice University in Houston and researchers from Ningbo University in China supports the structural models proposed in this research paper, and suggest that these two borophene sheets are likely to be superconducting at temperatures around 10K. These sheets in this study are relatively stable and loosely bound to the silver surface, properties that make them good candidates for eventual practical use for boron-based electronic devices.
More information: Baojie Feng et al. Experimental realization of two-dimensional boron sheets, Nature Chemistry (2016). DOI: 10.1038/nchem.2491
Abstract
A variety of two-dimensional materials have been reported in recent years, yet single-element systems such as graphene and black phosphorus have remained rare. Boron analogues have been predicted, as boron atoms possess a short covalent radius and the flexibility to adopt sp2 hybridization, features that favour the formation of two-dimensional allotropes, and one example of such a borophene material has been reported recently. Here, we present a parallel experimental work showing that two-dimensional boron sheets can be grown epitaxially on a Ag(111) substrate. Two types of boron sheet, a β12 sheet and a χ3 sheet, both exhibiting a triangular lattice but with different arrangements of periodic holes, are observed by scanning tunnelling microscopy. Density functional theory simulations agree well with experiments, and indicate that both sheets are planar without obvious vertical undulations. The boron sheets are quite inert to oxidization and interact only weakly with their substrate. We envisage that such boron sheets may find applications in electronic devices in the future.
Journal information: Nature Chemistry
© 2016 Phys.org



















