A new protein spin labelling technique
Site-directed spin labelling (SDSL) used in combination with electron paramagnetic resonance (EPR) spectroscopy has been a tried and trusted technique for elucidating the structure, function and dynamics of proteins and protein complexes. Nitroxide-based spin labels are among the most popular and best established ones because they are small, non-disturbing and exhibit excellent spectroscopic properties. "Ideal spin labelling procedures exhibit high reaction rates and selectivity," explains Professor Malte Drescher, Professor for Spectroscopy of Complex Systems at the University of Konstanz's Department of Chemistry and main author on the study alongside Professor Valentin Wittmann, who specializes in organic synthesis.
"Achieving high reactivity and high selectivity both at the same time can be a problem," continues Drescher. "Conventional spin labels based, for instance, on Gadolinium(III) or trityl, display either very broad spectra and low modulation depths or very narrow spectra that are unsuitable for the kinds of experiments that we want to conduct." A new study published by Drescher, Wittmann and their team of University of Konstanz chemists, which was published online in the journal ChemBioChem Communications on 14 August 2019, introduces a new approach for labelling proteins that features nitroxide-based spin labels and genetically encoded noncanonical amino acids (ncAAs) as targets for SDSL.
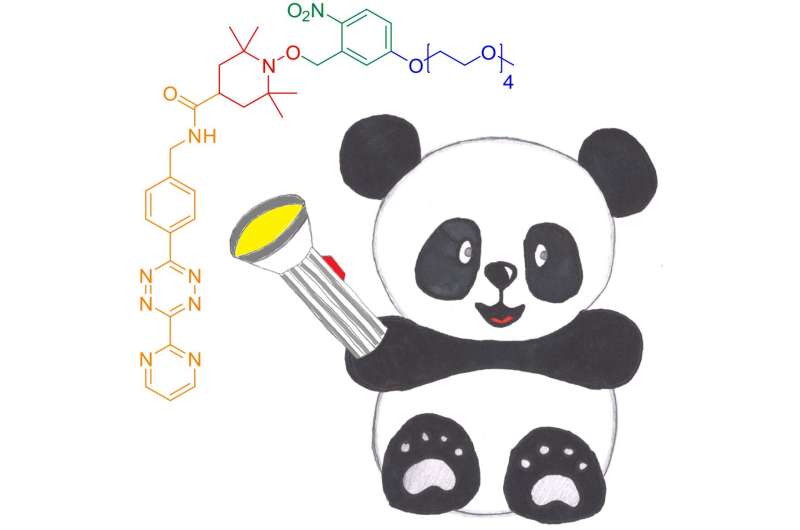
"Nitroxides provide ideal spectral width and access to dynamic information," says Anandi Kugele, a doctoral researcher at the Konstanz Research School of Chemical Biology (KoRS-CB) and first author on the study, who received a prestigious travel grant from the National High Magnetic Field Laboratory to present the results at the 2019 Rocky Mountain Conference on Magnetic Resonance in Denver, Colorado (USA). "Traditional nitroxide-based labels have limited redox stability, which is a drawback for in-cell applications. The challenge for us was to increase nitroxide stability and thus to adapt nitroxide-based spin labels for future routine in vivo use." To that end, the researchers developed a new spin label that can be attached to proteins by means of inverse-electron-demand Diels-Alder (DAinv) cycloaddition to genetically encoded ncAAs, a method which has proven suitable to a broad range of in vitro and in vivo applications. To achieve nitroxide stability, the researchers further used a protection strategy based on photoremovable protecting groups, which are known to protect nitroxides and to release them as needed.
The new spin label—termed photoactivatable nitroxide for DAinv reaction, or PaNDA for short—is water soluble, EPR-active and deprotection-efficient as both in vitro and in lysate tests with the two model proteins green fluorescent protein (GFP) and Escherichia coli oxidoreductase thioredoxin (TRX), which is found in virtually all known organisms, suggest. "We do need to improve on the method used to deliver the PaNDA spin label to cells and to test labelling and deprotection efficiencies inside the cell, amongst other things," concludes Malte Drescher: "But our research clearly demonstrates that, in principle, the PaNDA label can be used for EPR measurements in challenging biological environments, including the inside of cells. Our tests with E. coli lysate are very promising in this respect. This will open up a whole new range of opportunities for the study of proteins by means of EPR spectroscopy."
More information: Anandi Kugele et al, Protein Spin Labeling with a Photocaged Nitroxide Using Diels–Alder Chemistry, ChemBioChem (2019). DOI: 10.1002/cbic.201900318
Journal information: ChemBioChem
Provided by University of Konstanz