Team discovers protein, lipid connection that could aid new influenza therapies
The connection between an influenza virus surface protein and a host cell lipid has been discovered by researchers at the University of Maine and the National Institutes of Health. Confirmation of direct interaction between the protein and lipid could lead to new antiviral therapies.
The UMaine-led research team is now testing a hypothesis that a certain region within the protein hemagglutinin (HA)—its cytoplasmic tail—could be the site of interaction with the host cell lipid PIP2. Because of the stability of the HA tail, there is potential for a targeted treatment that could continue to work, despite the frequent mutations of other parts of HA, according to the scientists, who reported their findings in the Biophysical Journal.
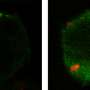
"Our findings show for the first time a connection between the influenza virus surface protein HA (the H in H1N1) and the host cell lipid PIP2," says UMaine professor of physics Samuel Hess, the team's lead scientist. "With further single-molecule microscopy experiments, we are now testing the hypothesis that a certain region within HA could be the site of interaction with PIP2."
HA has two roles, according to the Centers for Disease Control and Prevention website. The surface protein allows a flu virus to enter a healthy cell and acts as an antigen that can trigger an immune response that protects the host from reinfection by the same flu strain. That makes HA one of the active components of inactivated flu vaccines. According to the CDC, most seasonal flu vaccines are designed to target HA of the flu viruses that research suggests will be most common during flu season.
PIP2 controls a large number of cellular functions through signaling pathways it can modulate. Many of these pathways control the actin cytoskeleton, a structural framework for cell shape, motility and membrane organization. During flu infection, manipulation of such signaling pathways by the virus can allow it to suppress innate immune responses, keep infected cells alive, and increase the rate of assembly and escape of new viral particles.
Many proteins that have been seen together with HA are known to control the actin cytoskeleton, and they also have known binding to PIP2, but the connection was not previously explained.
Using confocal and super-resolution microscopy, the latter a patented technology developed by Hess, the researchers imaged HA and PIP2 in several living cell types and observed that they sometimes occupied the same regions in the plasma membrane defining the cell exterior. HA and PIP2 also were observed affecting each other's motions. Having HA present caused PIP2 to move more slowly, reverse direction more frequently, and be more highly confined into clusters. Having PIP2 present caused the density of HA to increase. A high density of HA on the surface of the virus is necessary for viral entry into uninfected cells through a process called membrane fusion.
More information: Nikki M. Curthoys et al, Influenza Hemagglutinin Modulates Phosphatidylinositol 4,5-Bisphosphate Membrane Clustering, Biophysical Journal (2019). DOI: 10.1016/j.bpj.2019.01.017
Journal information: Biophysical Journal
Provided by University of Maine