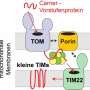
How proteins become embedded in a cell membrane
Many proteins with important biological functions are embedded in a biomembrane in the cells of humans and other living organisms. But how do they get in there in the first place? Researchers in the Department of Biosystems Science and Engineering at ETH Zurich have investigated the matter.
Nearly one-third of all proteins in living beings are firmly embedded in a biomembrane—either in a cell's outer membrane or in the boundaries of internal cellular compartments. There, these membrane proteins perform important tasks, serving, for instance, as molecular channels for transporting metabolites and nutrients through the membrane or as sensor proteins for sensing the cellular environment.
A team of researchers led by Daniel J. Müller, a professor in the Department of Biosystems Science and Engineering at ETH Zurich in Basel, has now investigated how membrane proteins manage to get into the membranes. To do this, they used a highly precise method that enables them to extract individual proteins from, or deposit them on, membranes. This method, known as single-molecule force spectroscopy, lets scientists guide a computer-controlled cantilever measuring just a few nanometers in thickness to a specific location on a membrane's surface with utmost precision. Molecular adhesive forces cause a protein located there to adhere to the cantilever.
Role of two helper proteins
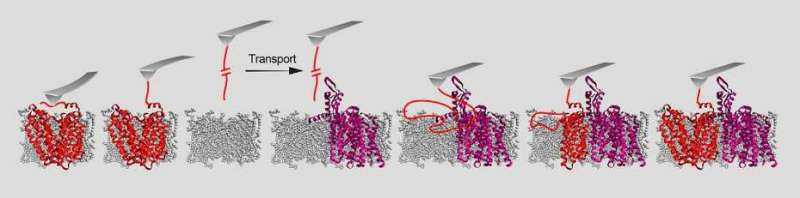
In experiments with bacterial proteins, the researchers were able to clarify the role of two helper proteins—an insertase and a translocase—that enable the membrane proteins to embed themselves in the membrane. Insertase is a single protein, while translocase is a complex composed of multiple proteins. Both of them ensure that a pore opens up in the membrane. "In the case of insertase, we can think of this pore as a slide. The membrane protein is initially present as an unstructured peptide strand that slips down this slide into the membrane. In the membrane, this peptide strand then takes on its functional three-dimensional shape," explains ETH Professor Müller. "Once the membrane protein has successfully become three-dimensional and embedded itself in the membrane, the helper protein detaches and forms a slide at a different location in the membrane for the next protein," he continues.
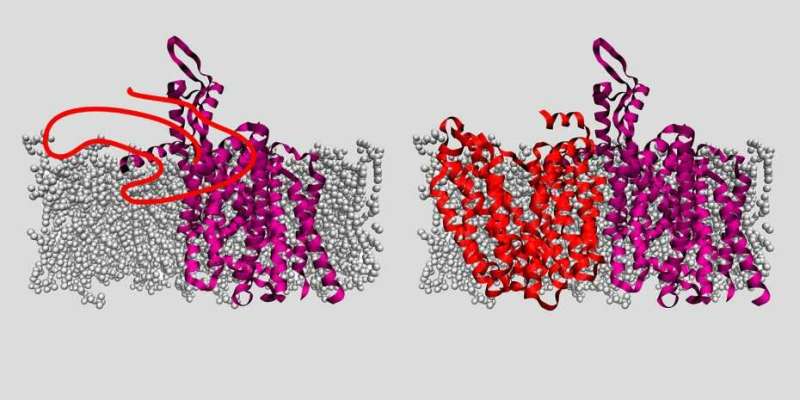
Up to now, research into how these helper proteins function was imprecise and used only short peptides or was conducted only outside of biomembranes. "We have now observed and described for the first time, step by step, how an entire protein embeds itself in a membrane and takes on a three-dimensional form," says Tetiana Serdiuk, a postdoc in ETH Professor Müller's group and the study's first author.
The ETH researchers were also able to show the differences in how insertases and translocases work: insertases insert peptide strands into the membrane relatively quickly but clumsily. "This means they work well, particularly with small proteins," says Müller. Translocases, on the other hand, insert peptide strands into the membrane section by section, making them better suited for more complex proteins.
Important for medicine
This study is a case of classic basic research, which is particularly significant in view of the importance of membrane proteins to medicine, as Müller emphasises: "Around half of all drugs act on membrane proteins, and we need to understand how these membrane proteins form and how they work."
In addition, single-molecule force spectroscopy, which the ETH scientists further refined for this study, could be used in other applications: in connection with the National Center of Competence in Research (NCCR) for Molecular Systems Engineering, Müller and other scientists are working to develop artificial biological cells. "This method could be used to custom-fit biomembranes with proteins, essentially programming them," says the ETH professor. "Artificial cells of this kind could one day be used as molecular factories to produce pharmaceuticals on an industrial scale."
More information: Serdiuk T et al. Insertion and folding pathways of single membrane proteins guided by translocases and insertases. Science Advances 2019, 5: eaau6824, DOI: 10.1126/sciadv.aau6824 , advances.sciencemag.org/content/5/1/eaau6824
Journal information: Science Advances
Provided by ETH Zurich