Chemist creates nanoreactors to synthesize organic substances under visible light
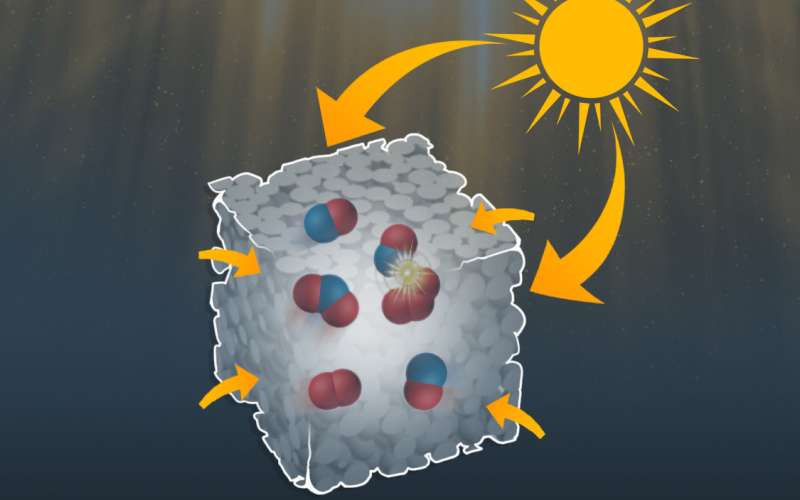
A RUDN chemist has developed new photocatalysts consisting of nanostructures from titanium dioxide. Hollow nanocubes with ultra-thin walls act like nanoreactors and provide for 28-fold more effective organic reactions at room temperature under the influence of visible light. The results are published in Applied Catalysis B: Environmental.
Traditional methods of manufacturing pharmaceuticals, fertilizers, pesticides, food additives, and other useful products from organic substances require high pressure and temperature levels. Photocatalysis is a highly efficient process for chemical production. Photocatalysts speed up organic reactions under the influence of light in ambient conditions without increasing temperature or pressure.
Titanium dioxide is considered a prospective catalyst. However, its catalytic activity is activated only in UV light, which comprises only 5 percent of sunlight. When shaped as hollow nanostructures, titanium dioxide becomes more active as a catalyst. Rafael Luque, the director of the Center for Molecular Design and Synthesis of Innovative Compounds for Medicine and colleagues from Iran describe a new type of structure with high photocatalytic activity: black hollow nanocubes made of titanium dioxide (BHC-TiO2).
The development of the new nanostructures took almost two years. The procedure consists of four steps. First, the chemists prepare nanocubes made of hematite and cover them with titanium dioxide. Then, the insides of the cubes are washed out using a solution of hydrochloric acid, leaving only the thin titanium dioxide shell. This is heated to 550°C in a hydrogen-argon atmosphere. After that, the samples turn into black hollow nanocubes. The whole process takes two to three days.
"The main advantages of our structures are that they are easy to create, durable, and can be used for different purposes. BHC-TiO2 can be used as a photocatalyst for water purification to accelerate the decomposition of pollutants, as well as for biomass conversion. Currently, we are studying the application of photocatalysts in the production of organic substances," said Luque.
In an experiment involving benzimidazole synthesis, the researchers checked the catalytic activity of several types of nanocubes—solid ones made of titanium dioxide, hollow ones, and baked black hollow BHC-TiO2 ones. Some samples were exposed to visible light from a regular halogenic lamp, and some—to UV radiation. The derivatives of this substance are in high demand in the pharmaceutical industry
BHC-TiO2 particles showed high catalytic activity under both types of exposure. Eighty-six percent of the initial substance was processed under the influence of visible light, which is 28 times more than in the experiment with one-piece (non hollow) titanium dioxide cubes. The chemists believe that this activity is due to the structural hollowness, large surface area, and porous ultra-thin walls. All these properties make nanocubes work as nanoreactors, i.e. reflect and scatter light and easily absorb organic substances, creating a medium for effective reactions inside the cubes. Ti3+ ions formed on the surface of nanocubes in the course of baking also play an important role. RUDN scientists believe they facilitate electron transfer making the whole structure absorb visible light (and not just the UV light like pure titanium dioxide).
The experiments proved a high durability of the nanoreactors—even after the sixth use, the structures kept their form and almost all Ti3+ ions on their surface. Therefore, BHC-TiO2 can be used to carry out at least 7 organic reactions without any loss in their catalytic activity.
More information: Abolfazl Ziarati et al, Black hollow TiO2 nanocubes: Advanced nanoarchitectures for efficient visible light photocatalytic applications, Applied Catalysis B: Environmental (2018). DOI: 10.1016/j.apcatb.2018.07.020
Provided by RUDN University