Chemists convert titanium nanoparticles into an efficient weapon against pollution
Researchers from RUDN University (Russia) have come up with a new method to convert titanium nanoparticles into an efficient substance capable of removing toxic phenol from water, even in visible light. The results of the study are reported in the Journal of Materials Science: Materials in Electronics.
"Environmental pollution is arguably one of the greatest threats to humanity—and to the whole planet Earth. Industry is responsible for many types of pollution, and the transfer of toxic organic substances into the water is one of them," says Yahya Absalan, Ph.D student at RUDN and the lead author of the paper. One such substance is phenol (and its derivatives), produced on a large scale (about 7 billion kilos per year). Phenol derivatives act as precursors to many materials and compounds, plastics, detergents and pharmaceuticals. However, this substance may cause harmful effects on the central nervous system and heart, the liver and kidneys.
Chemists have elaborated quite a few methods to remove phenol from water. One of these methods considers nanomaterials, valued for their optical and magnetic properties. These properties come from unique features of nanoparticles due to their large surface and surface activity.
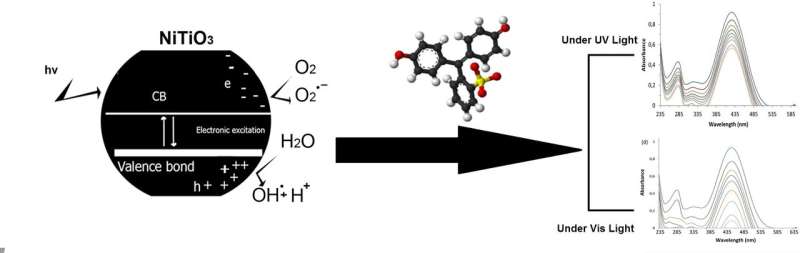
The researchers from RUDN University worked with titanium dioxide (TiO2) nanoparticles. This is a semiconductor that, after absolving UV-light, is capable of reacting with water. This reaction leads to the production of two radicals—OH• and O2-•, that, in turn, may react with phenol and reduce the concentration of phenol in water.
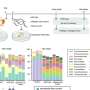
However, the energy band gap of dioxide titanium is 3.25, so it may only absorb UV-light (and, therefore, purify water only under very specific and costly conditions). The RUDN chemists tried to modify dioxide titanium with transition metals—metallic elements occupying a central block (Groups IVB–VIII, IB, and IIB, or 4–12) in the periodic table. Pure MTiO3 ("M" here stands for "metal"—cadmium, chrome, nickel, manganese, iron and cobalt) nanoparticles were formed when the precursor was heat-treated at 750 °C for 210 minutes.
Doping a transition metal into dioxide titanium allows researchers to reduce the energy band gap and, consequently, increase the wavelength. TiO3 can absorb not only UV-light, but visible light, as well. This would allow researchers to remove phenol from water in non-specific conditions almost everywhere.
The researchers have already planned to expand the scope of their experiment by using rare metals instead of transitional metals, for instance. "There is still a long way to go before we could transfer our new method to industry. Six months, or two years, perhaps. Anyway, this is a very promising substance to be used against pollution," concluded Absalan.
More information: Yahya Absalan et al, Novel synthesis method for photo-catalytic system based on some 3d-metal titanates, Journal of Materials Science: Materials in Electronics (2017). DOI: 10.1007/s10854-017-7769-6
Provided by RUDN University