A novel catalyst for high-energy aluminum-air flow batteries
A recent study affiliated with UNIST has introduced a novel electric vehicle (EV) battery technology that is more energy efficient than gasoline-powered engines. The new technology involves replacing battery packs instead of charging them, bypassing the slow-charging issues of existing EV battery technology. It also provides lightweight, high-energy density power sources with little risk of burning or explosion. This breakthrough has been led by Professor Jaephil Cho and his research team in the School of Energy and Chemical Engineering at UNIST. Their findings have been published in Nature Communications.
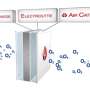
The researchers developed a new type of aluminum-air flow battery for EVs. The new battery outperforms existing lithium-ion batteries in terms of higher energy density, lower cost, longer cycle life, and higher safety. Aluminum-air flow batteries are primary cells, which means they cannot be recharged via conventional means. In EVs, they produce electricity by replacing the aluminum plate and electrolyte. Considering the actual energy density of gasoline and aluminum of the same weight, aluminum is superior.
"Gasoline has an energy density of 1,700 Wh/kg, while an aluminum-air flow battery exhibits a much higher energy density of 2,500 Wh/kg with its replaceable electrolyte and aluminum," says Professor Cho. "This means with 1kg of aluminum, we can build a battery that enables an electric car to run up to 700km."
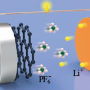
The new battery works much like metal-air batteries, producing electricity from the reaction of oxygen in the air with aluminium. Metal-air batteries, especially aluminium-air batteries, have attracted much attention as the next-generation battery due to their energy density higher than that of LIBs. Indeed, batteries that use aluminum, a lightweight metal, are lighter, cheaper, and have a greater capacity than a traditional LIB.
Despite their high energy density, aluminum-air batteries are not widely used because of problems with high anode cost and byproduct removal when using traditional electrolytes. Professor Cho has solved this issue by developing a flow-based aluminum-air battery to alleviate the side reactions in the cell, where the electrolytes can be continuously circulated.
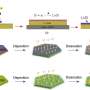
In the study, the research team prepared a silver nanoparticle seed-mediated silver manganate nanoplate architecture for the oxygen reduction reaction (ORR). They discovered that the silver atom can migrate into the available crystal lattice and rearrange the manganese oxide structure, thus creating abundant surface dislocations. Thanks to improved longevity and energy density, the team anticipates that their aluminum-air flow battery system could potentially bring more EVs to the road with greater range and substantially less weight with zero risk of explosion.
"This innovative strategy prevented the precipitation of solid by-product in the cell and dissolution of a precious metal in air electrode," says Jaechan Ryu, first author of the study. "We believe that our AAFB system has the potential for a cost-effective and safe next-generation energy conversion system."
The discharge capacity of aluminum-air flow battery is 17 times that of conventional aluminum air batteries. Additionally, the capacity of newly developed silver-manganese oxide-based catalysts was comparable to that of the conventional platinum catalysts (Pt/C). As silver is 50 times less expensive than platinum, it is also competitive in terms of the price
More information: Jaechan Ryu et al, Seed-mediated atomic-scale reconstruction of silver manganate nanoplates for oxygen reduction towards high-energy aluminum-air flow batteries, Nature Communications (2018). DOI: 10.1038/s41467-018-06211-3
Journal information: Nature Communications